Electrolyte compositions for batteries using sulphur or sulphur compounds
a technology of electrolyte composition and sulphur compound, which is applied in the direction of electrochemical generators, non-aqueous electrolyte accumulator electrodes, non-aqueous electrolyte accumulator electrodes, etc., to achieve the effect of increasing the cycle life of the battery, increasing the cycling efficiency and temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
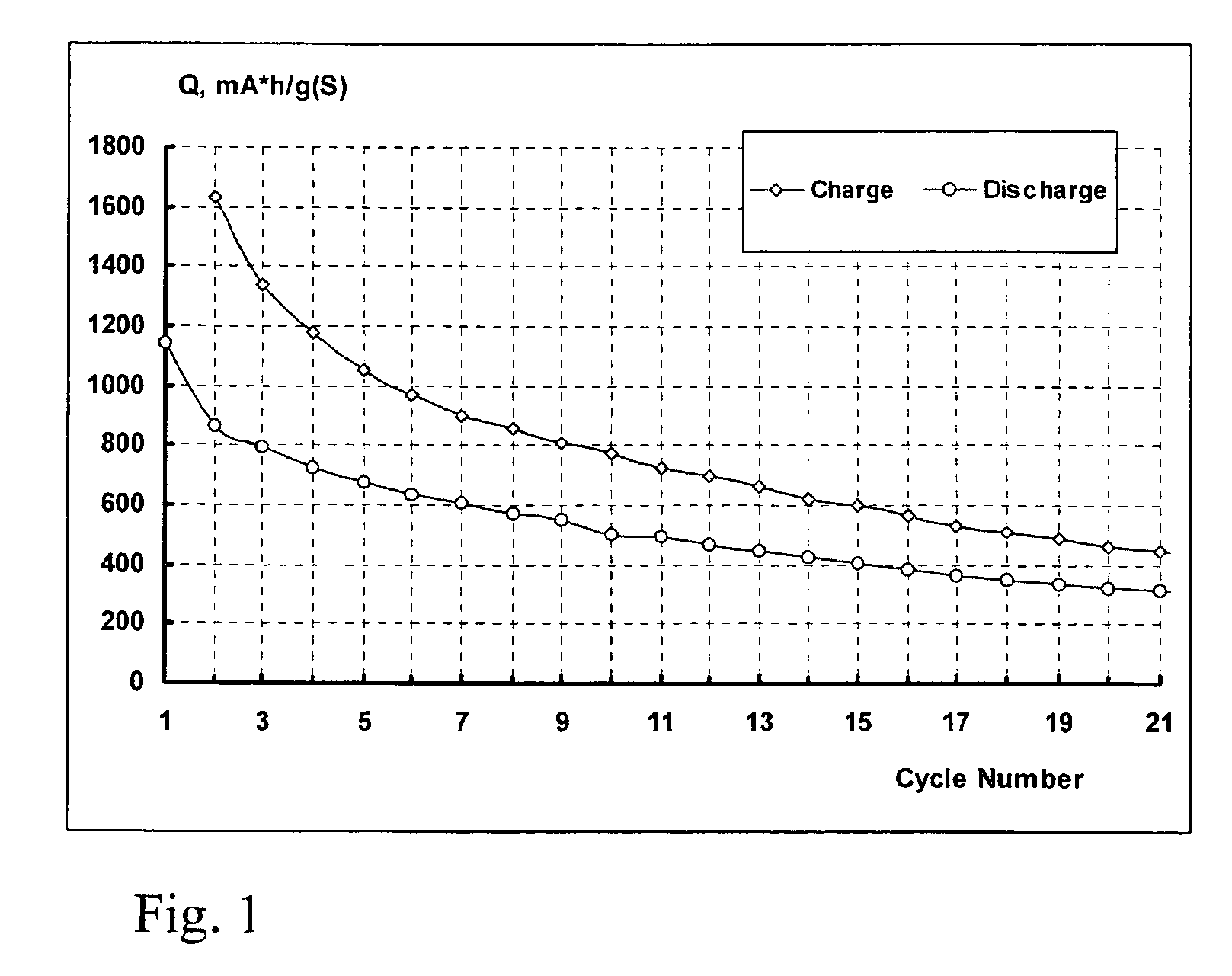
[0060] A lithium-sulphur cell was produced by assembling an anode made of metal lithium foil; a porous separator Celgard 2500 (a registered trademark of Celgard Inc., available from Celgard K.K., Tokyo, Japan, and also available from Celgard Inc. South Lakes, N.C. USA.); and a sulphur cathode comprising elemental sulphur as a depolariser (70% by weight), a carbon electro-conducting additive (10% by weight) Ketjenblack EC-600JD (available from Akzo Nobel Polymer Chemicals BV, Netherlands), and a binder (polyethyleneoxide with molecular mass 4000000-20% by weight). The sulphur cathode was deposited by an automatic film applicator Elcometer SPRL onto one side of an 18 micrometer thick conductive carbon coated aluminium foil (available from InteliCoat®, South Hadley, Mass.) as a current collector and substrate. A specific surface capacity of the cathode was 1 mAh / cm2. The assembled cell was filled with an electrolyte comprising a 0.1M solution of LiClO4 in sulpholane. All stages of the ...
example 2
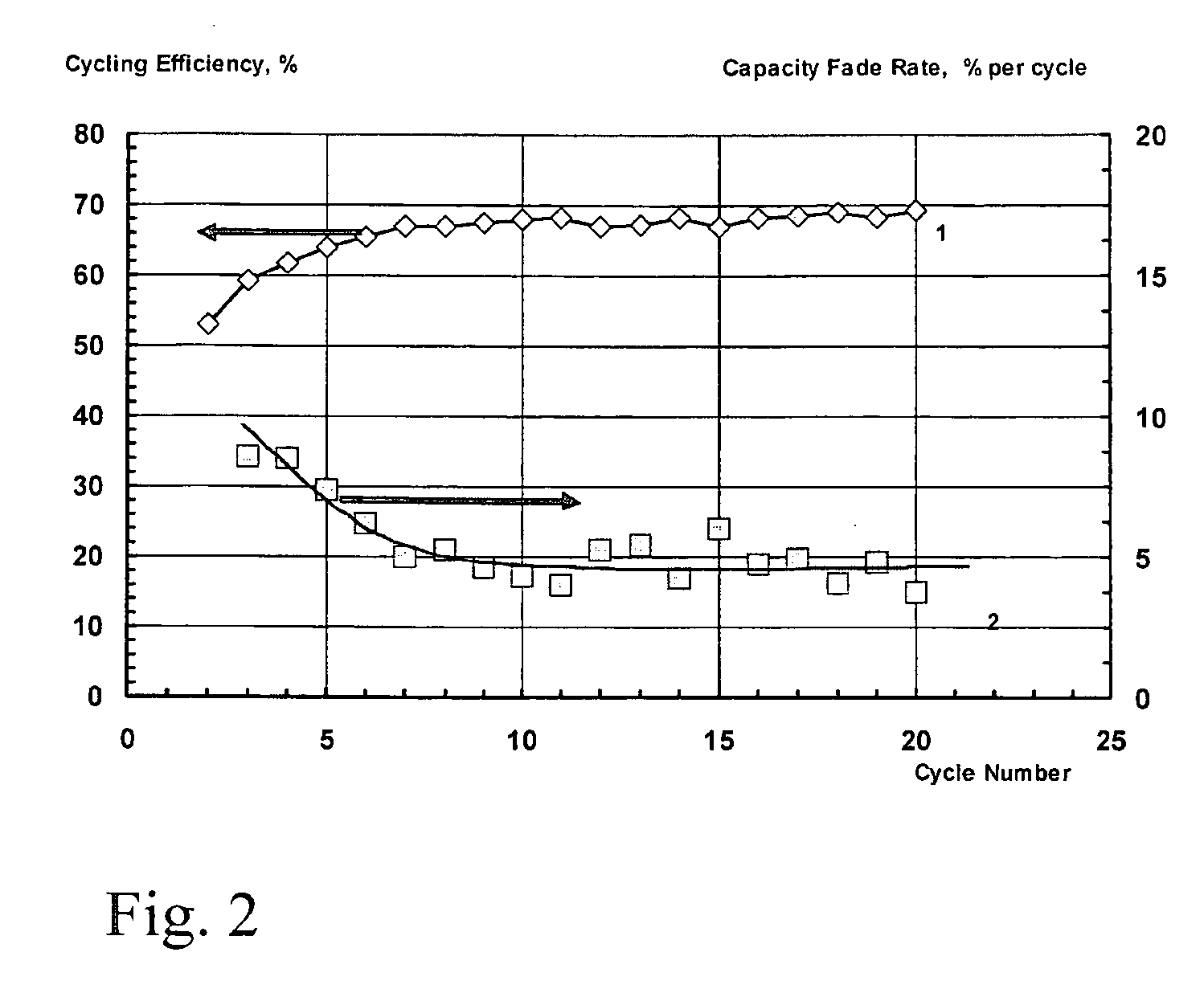
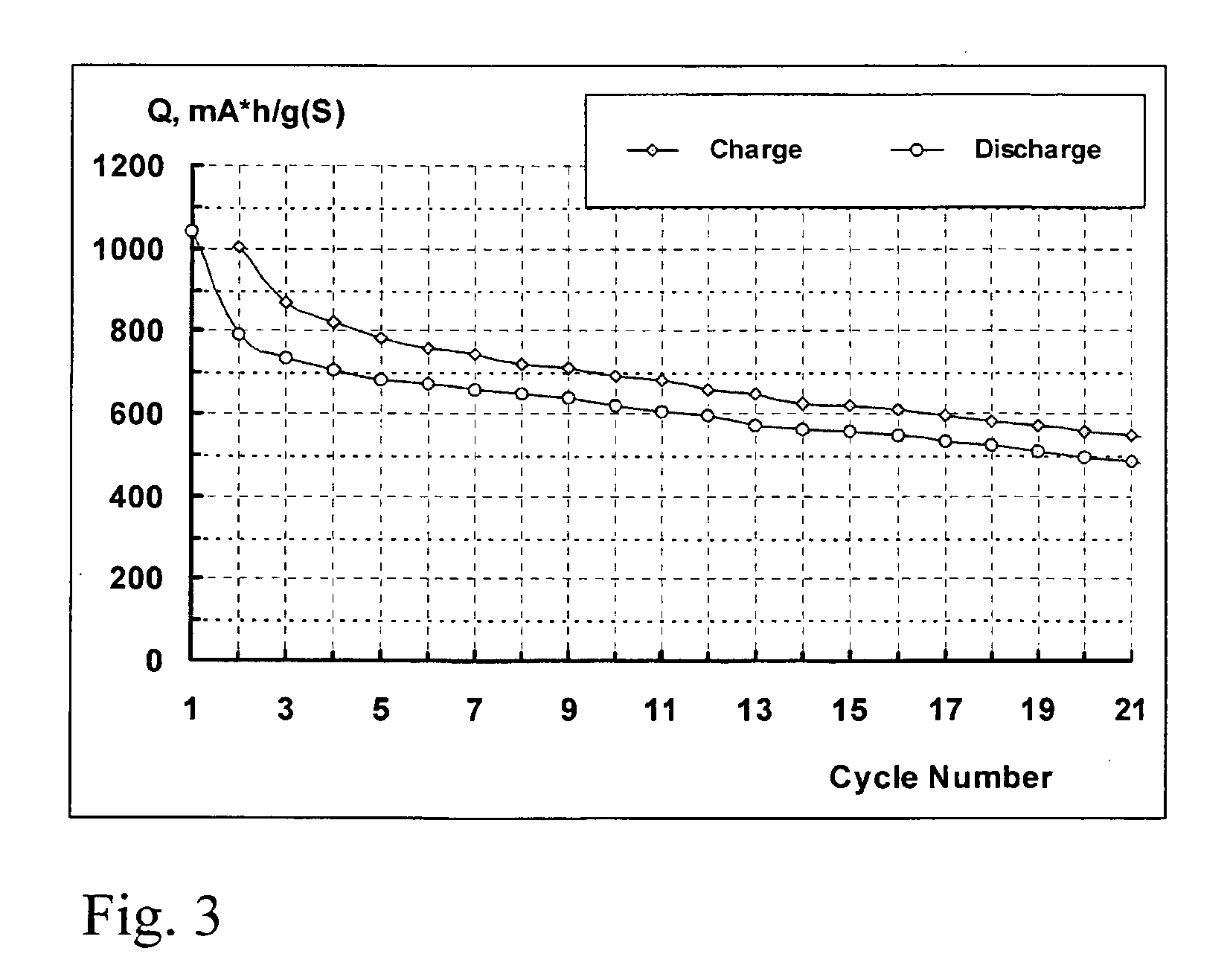
[0063] A lithium-sulphur cell was produced as described in the Example 1, but this time the assembled cell was filled with an electrolyte comprising a 1 M solution of LiClO4 in sulpholane. The cell was cycled at a charge and discharge rate of 0.25 C and at a temperature of 25° C. The change in the charge and discharge capacity of the cell during the cycling is shown in Figure, showing the capacity fade of the sulphur electrode in lithium-sulphur cell during cycling. In FIG. 3, the electrolyte is a 1 M solution of LiClO4 in sulpholane, the charge rate is 0.25 C, and the discharge rate is 0.25 C.
[0064] The change in the cycling efficiency and the rate of the capacity fade during cycling are shown in FIG. 4. In FIG. 4 the electrolyte is 1 M solution of LiClO4 in sulpholane.
[0065] As can be seen in FIG. 4, the efficiency of cycling and the rate of capacity fade initially change after the beginning of cycling, but later on they stabilize. The mean cycling efficiency between the 10th an...
example 3
[0066] A lithium-sulphur cell was produced as described in the Example 1, but this time the assembled cell was filled with an electrolyte comprising a 2M saturated solution of LiClO4 in sulpholane in accordance with an embodiment of the present invention. The cell was cycled at a charge and discharge rate of 0.25 C and at a temperature of 25° C. The change in the charge and discharge capacity of the cell during the cycling is shown in FIG. 5, showing the capacity fade of a sulphur electrode in a lithium-sulphur cell during cycling. In FIG. 5, the electrolyte is a 2 M solution of LiClO4 in sulpholane, the charge rate is 0.25 C, and the discharge rate is 0.25 C.
[0067] The change in the cycling efficiency and the rate of the capacity fade during cycling are shown in FIG. 6. In FIG. 6, the electrolyte is 2 M solution of LiClO4 in sulpholane. As can be seen in FIG. 6, the efficiency of cycling and the rate of capacity fade initially change after the beginning of cycling, but later on th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| charge rate | aaaaa | aaaaa |
| operating temperature | aaaaa | aaaaa |
| operating pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com