Computational method for designing enzymes for incorporation of amino acid analogs into proteins
a technology design methods, applied in the field of amino acid analog design and protein incorporation, can solve the problems of limited mutagenesis to the 20 naturally occurring amino acids, inability to circumvent the specificity of synthetases, and modest chemical functionality that has been accessed by this method, and achieve the effect of facilitating the accommodation of amino acid analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
I. Overview
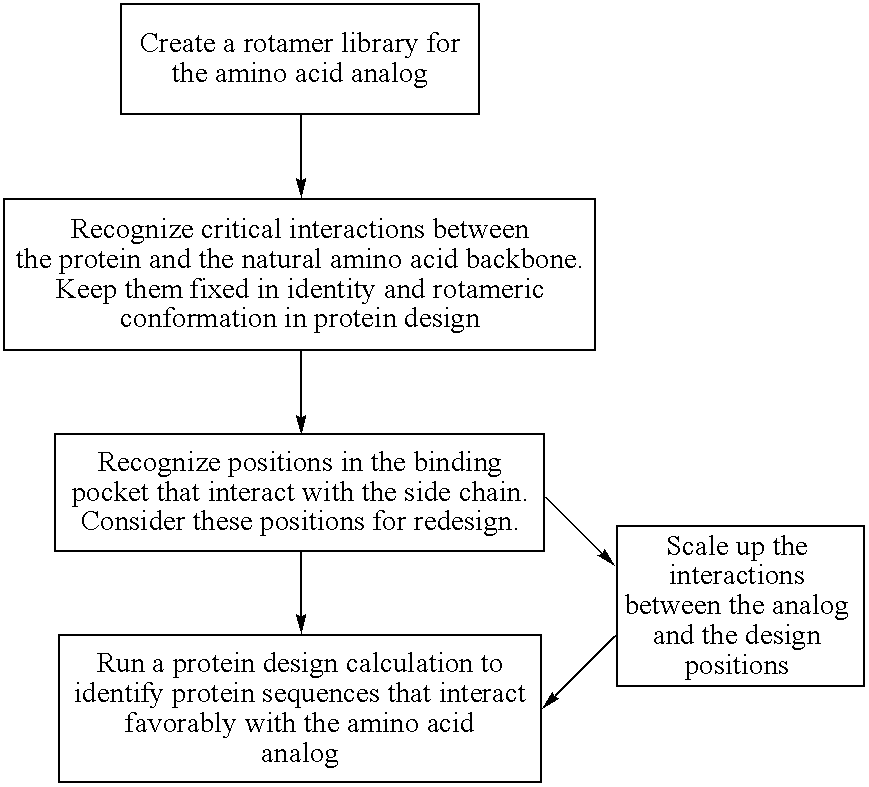
[0056] The instant invention provides methods and computational tools for modifying the substrate specificity of an AminoAcyl tRNA Synthetases (AARSs) through mutation to enable the enzyme to more efficiently utilize amino acid analog(s) in protein translation systems, either in vitro or in whole cells. A salient feature to the instant invention are methods and tools for systematically redesigning the substrate binding site of an AARS enzyme to facilitate the use of unnatural substrates in the peptide or protein translation reaction the enzyme catalyzes.
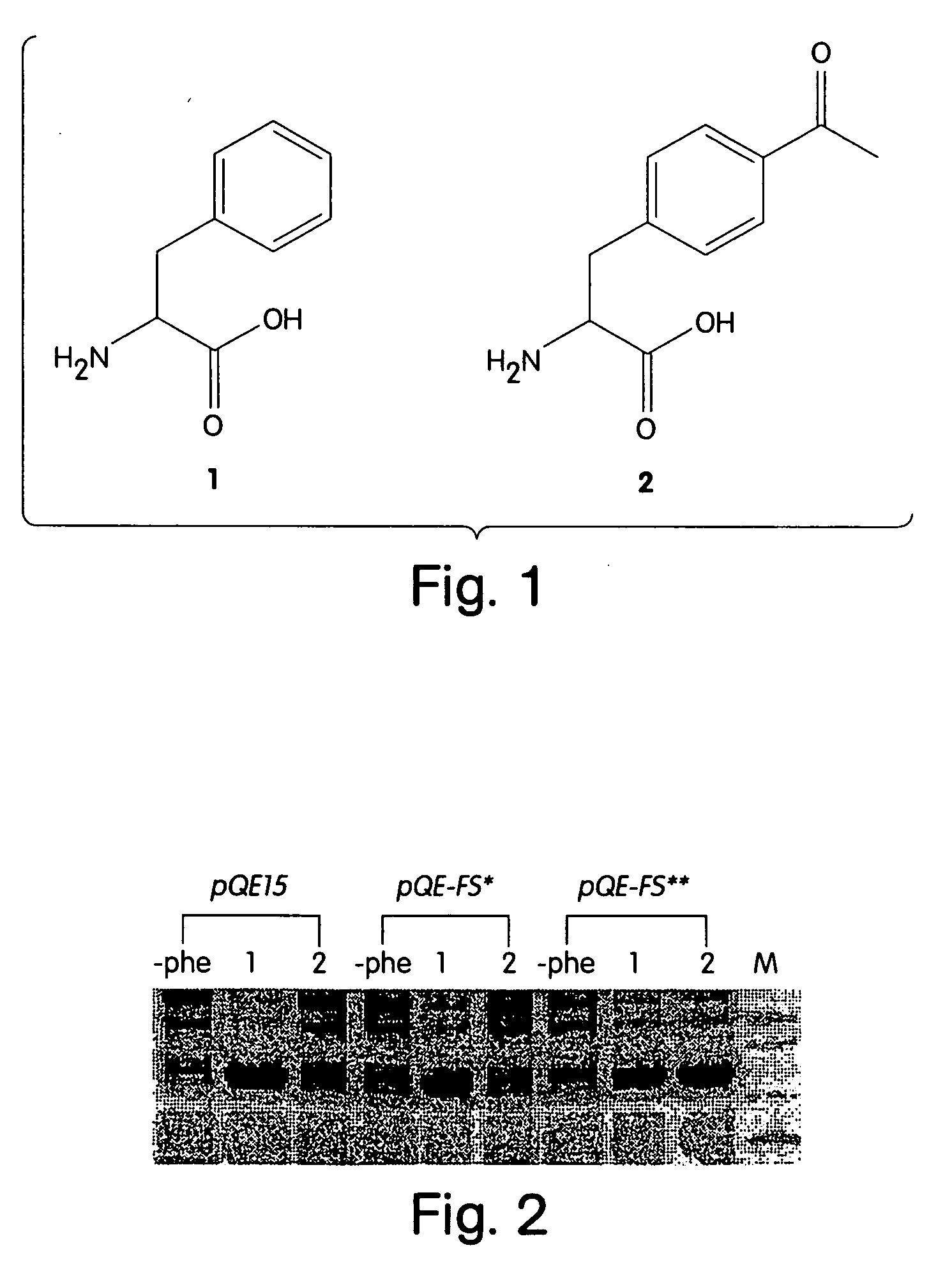
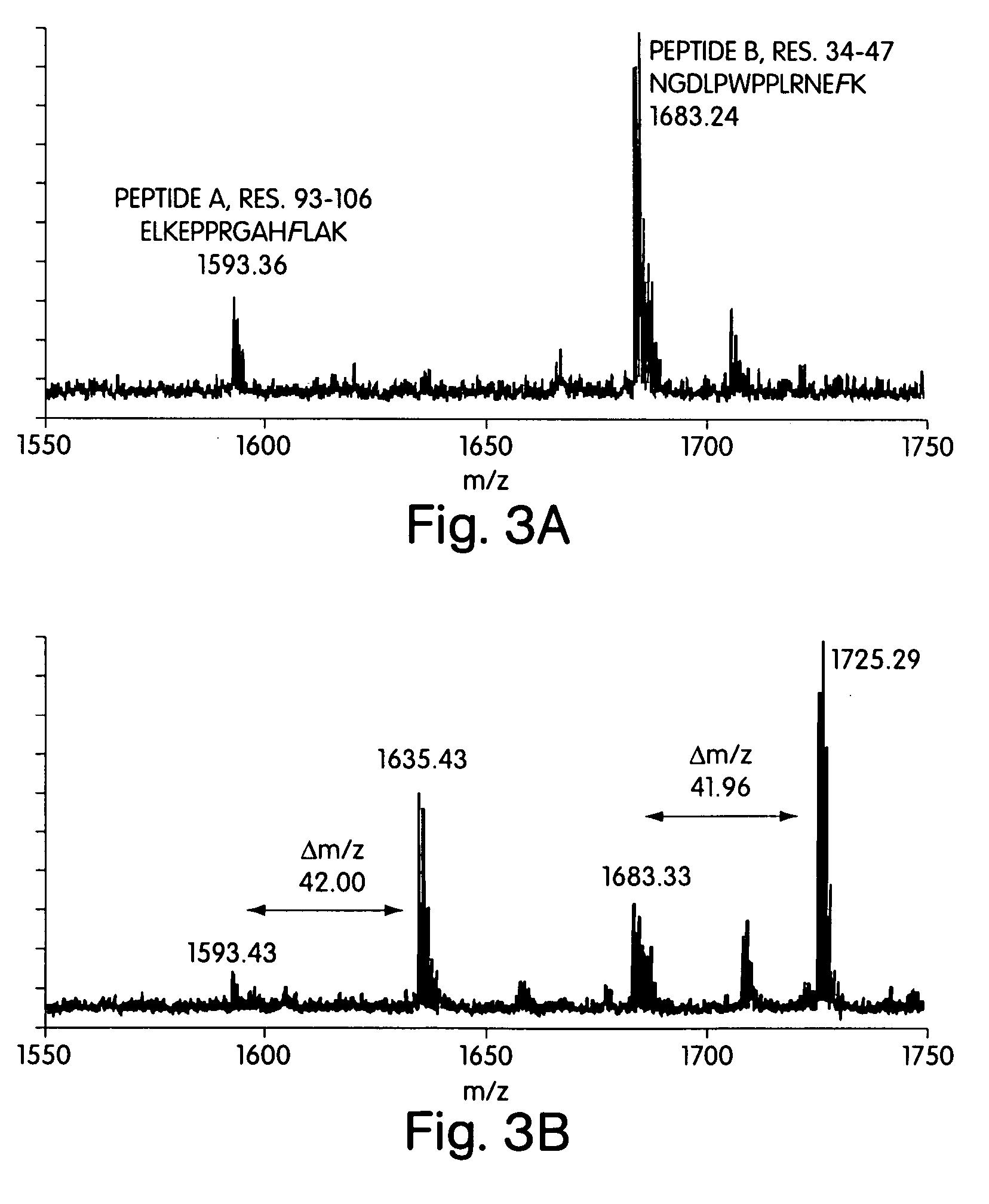
[0057] In one embodiment, the subject method uses a rotamer library for the intended amino acid analog (or “analog”), e.g., which represents the coordinates for a plurality of different conformations of the analog resulting from varying torsional angles in the molecule. Members of the rotamer library are modeled in a docking simulation with a model of an AARS, e.g., which includes the coordinates for the substrate binding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com