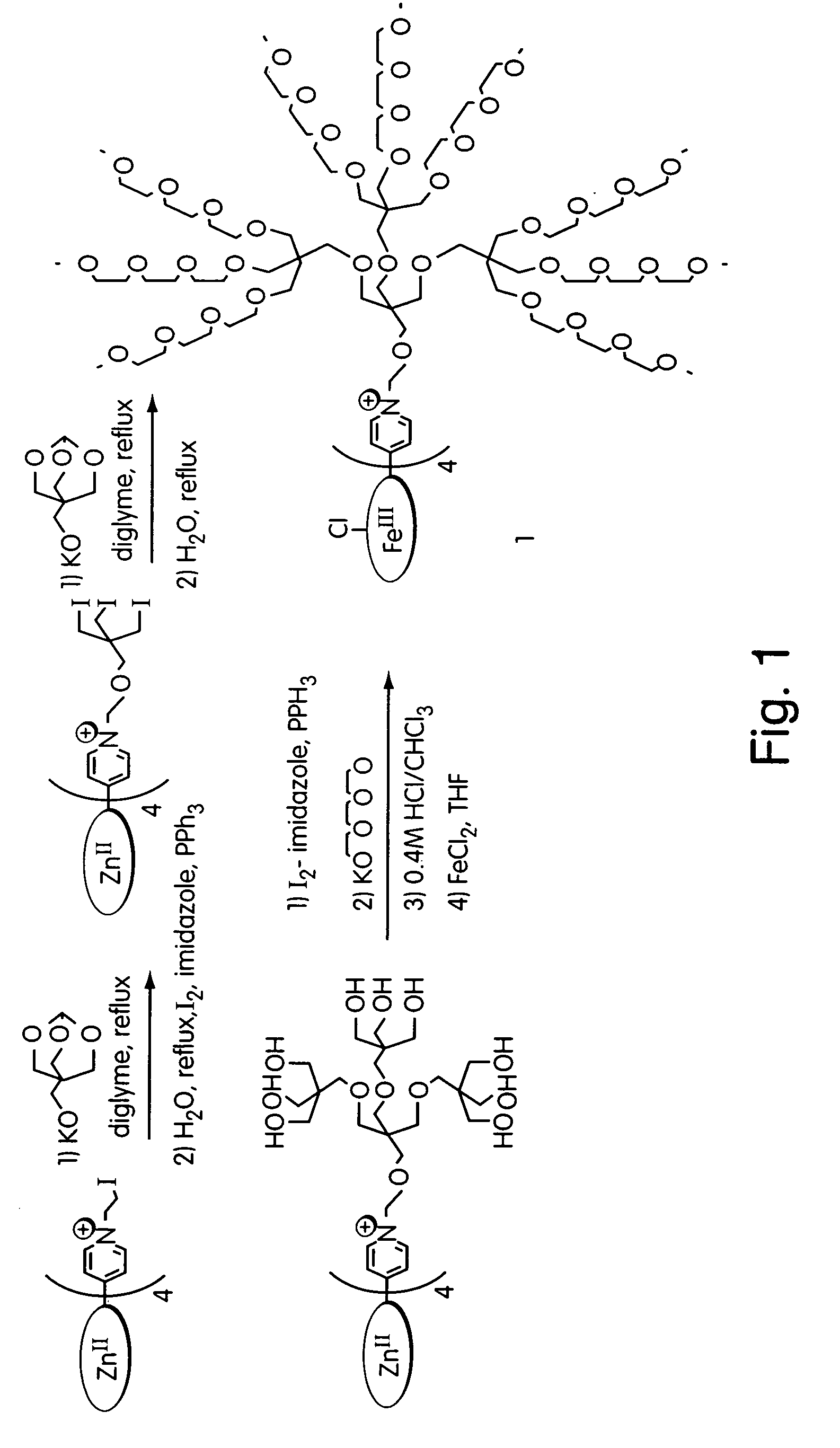
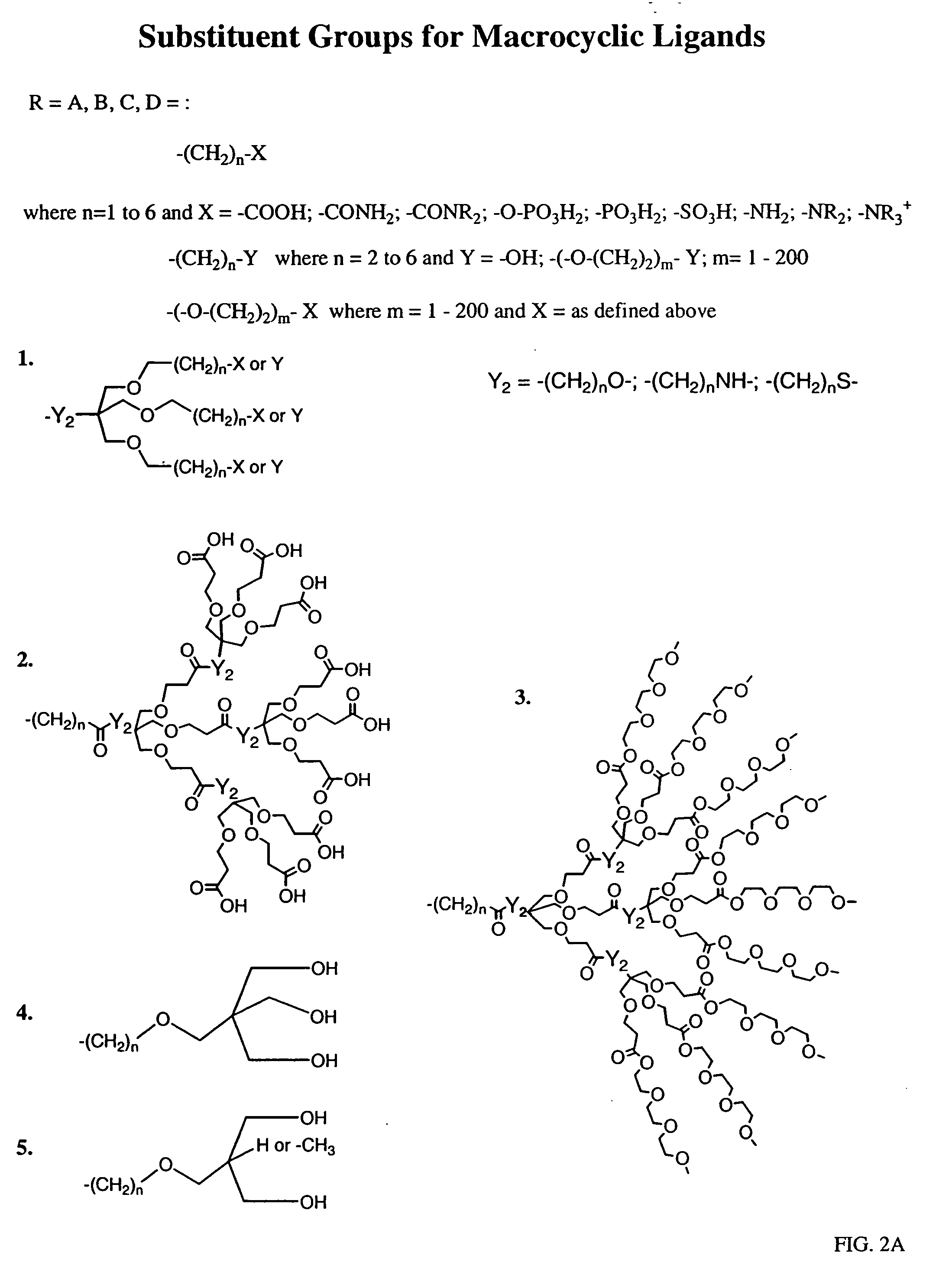
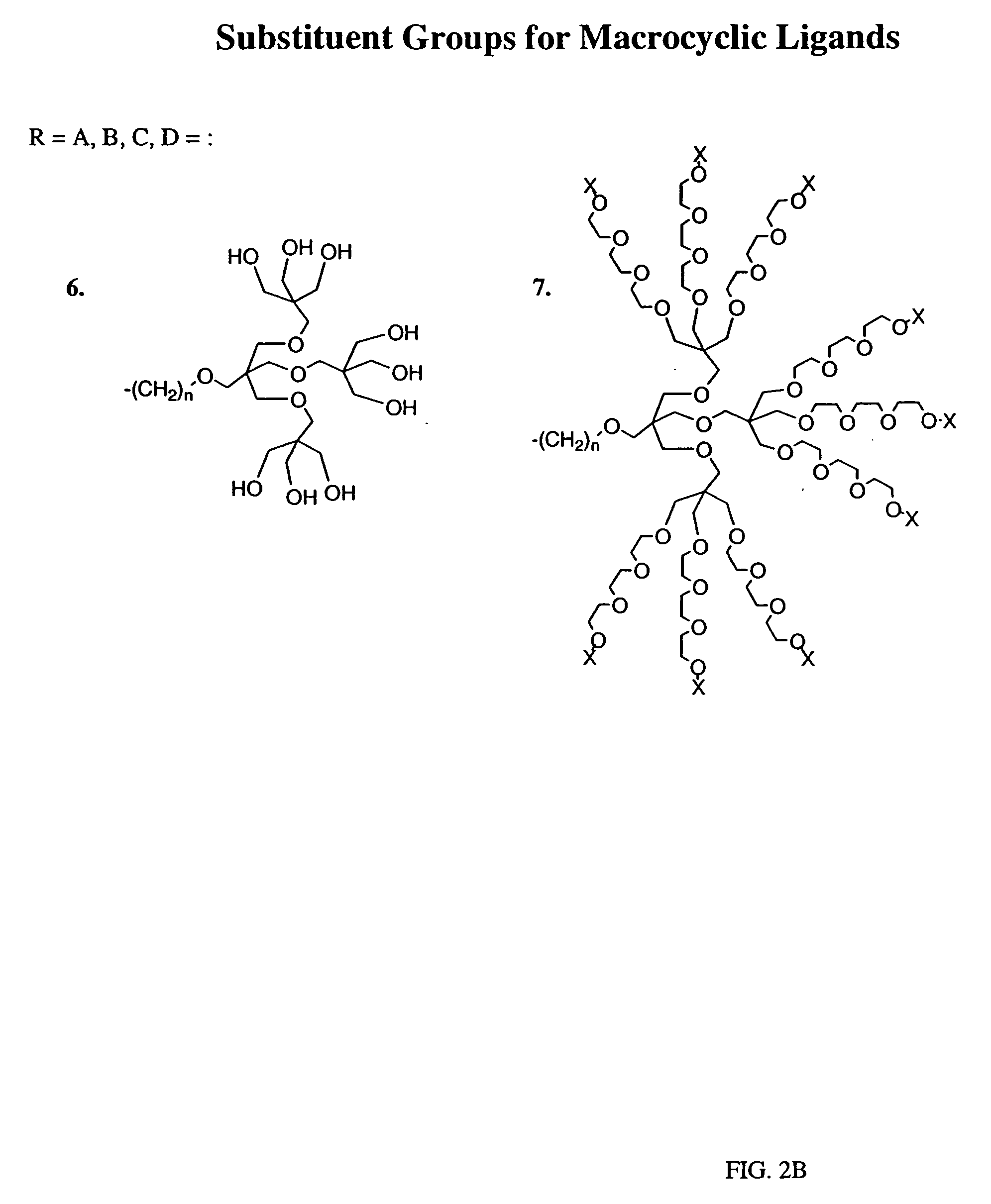
Peroxynitrite decomposition catalysts and methods of use thereof
a technology of peroxynitrite and catalyst, which is applied in the field of substituting porphyrin, porphyrazine, texaphyrin, or corrolemetal complex, can solve problems such as damage to biological targets, and achieve the effects of high catalytic activity, enhanced lifetime in the blood pool, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Tetrakis(allyl-2-pyridyl)porphyrin (VIII)
[0086] In an oven-dried flask under argon, 100 mg tetrakis(2-pyridyl)porphyrin (2-PyP) was dissolved in 5 mL allyl bromide, and reaction was heated at 100° C. for 16 hours. The reaction mixture was heated at 100° C. for 8 hours. Two methods were used to monitor the completion of the reaction. First, the shift of the porphyrin Soret band from λmax=412 nm (methanol) to λmax=418 nm (methanol) was monitored. A second method involved partition of an aliquot of the reaction mixture between H2O and CHCl3 whereby a complete reaction showed no color in the CHCl3 layer. Upon completion of the reaction, the allyl bromide was distilled off under high vacuum, and the residue was triturated with ethyl ether (Et2O). Chromatography was performed on Sephadex LH-20 (column dimensions were 2×20 cm) using methanol as the eluent and fractions that showed porphyrin with λmax=418 nm were retained. Compound VIII was produced as a shiny purple solid, in...
example ii
Synthesis of Compound (IX): Tetrakis(acetamido-2-pyridyl)porphyrin
[0087] In an oven-dried flask under argon, 50 mg 2-PyP was added to 1.0 g bromoacetamide and 2.5 mL dry DMF. The reaction mixture was heated at 100° C. for 8 hours. The reaction was monitored as above. Compound IX was isolated by dropping the completed reaction mixture into Et2O and precipitating the resulting solid from methanol / Et2O. The solid was filtered and washed well with CHCl3 and Et2O. Chromatography on Sephadex LH-20 (column dimensions 2×15 cm) using methanol as the eluent and by collecting the fastest-running red band produced (IX) in 75% yield. Absorbance data included the following: UV-Vis: λmax (run) (log10ε0) 418(5.20) 512(4.27) 587(4.01). ES-MS: Molecular ion at 850 (M-4 Br).
example iii
Synthesis of Compound (X): Tetrakis (ethylaceto-2-pyridyl)porphyrin
[0088] To an oven-dried flask under argon was added 10 mg 2-PyP, 1 mL ethyl bromoacetate, and 2 mL dry DMF. The reaction mixture was heated at 100° C. for 6 hours. The reaction was monitored by the shift in the porphyrin Soret band from λmax=412 nm (methanol) to λmax=420 nm (methanol), and, by, partition of an aliquot of the reaction mixture between H2O and CHCl3 where a complete reaction showed no color in the CHCl3 layer. When the reaction mixture was cooled to room temperature, some of the desired porphyrin precipitated out of solution. The remainder of product was precipitated from the reaction mixture by addition of Et2O, and precipitate was filtered and washed well with CHCl3 and Et2O. The brown solid was precipitated from methanol / Et2O and filtered to give compound (X), as purple crystals, in 75% yield. Absorbance data included the following: UV-vis: λmax (nm) (log10ε0) 420(5.29) 512(4.46) 555(4.30) 585(4.28)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| metallic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com