Stent deployment systems and methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
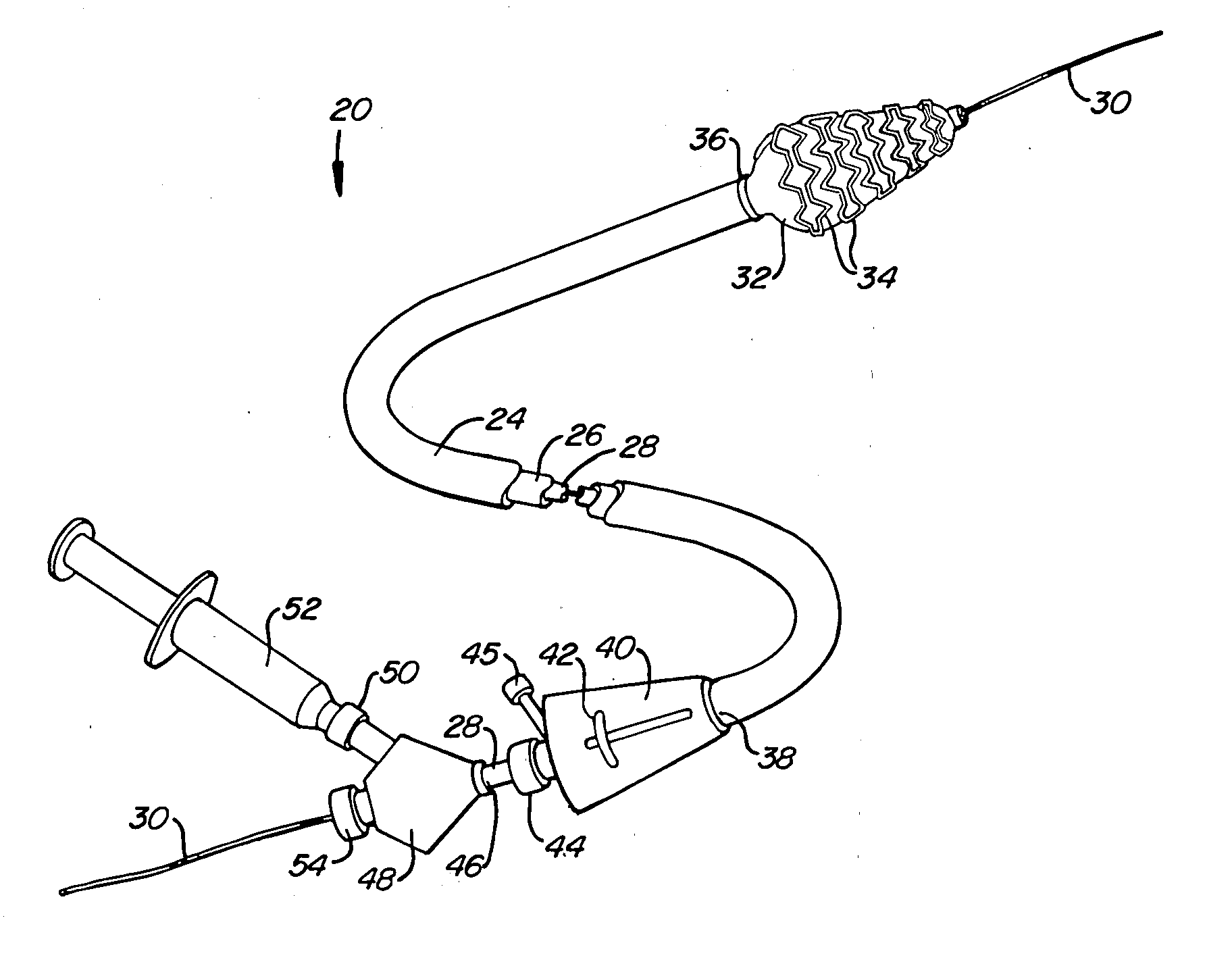
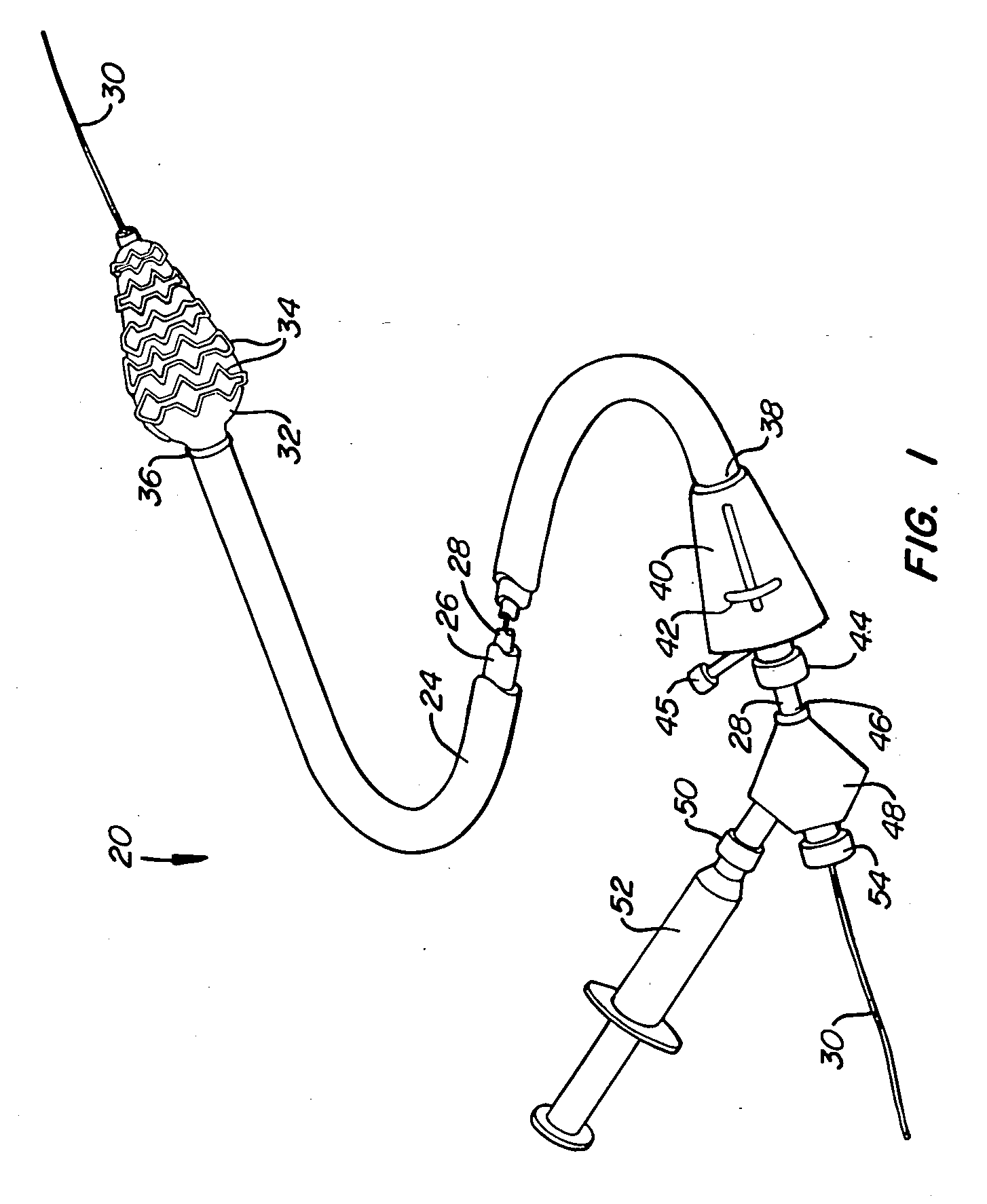
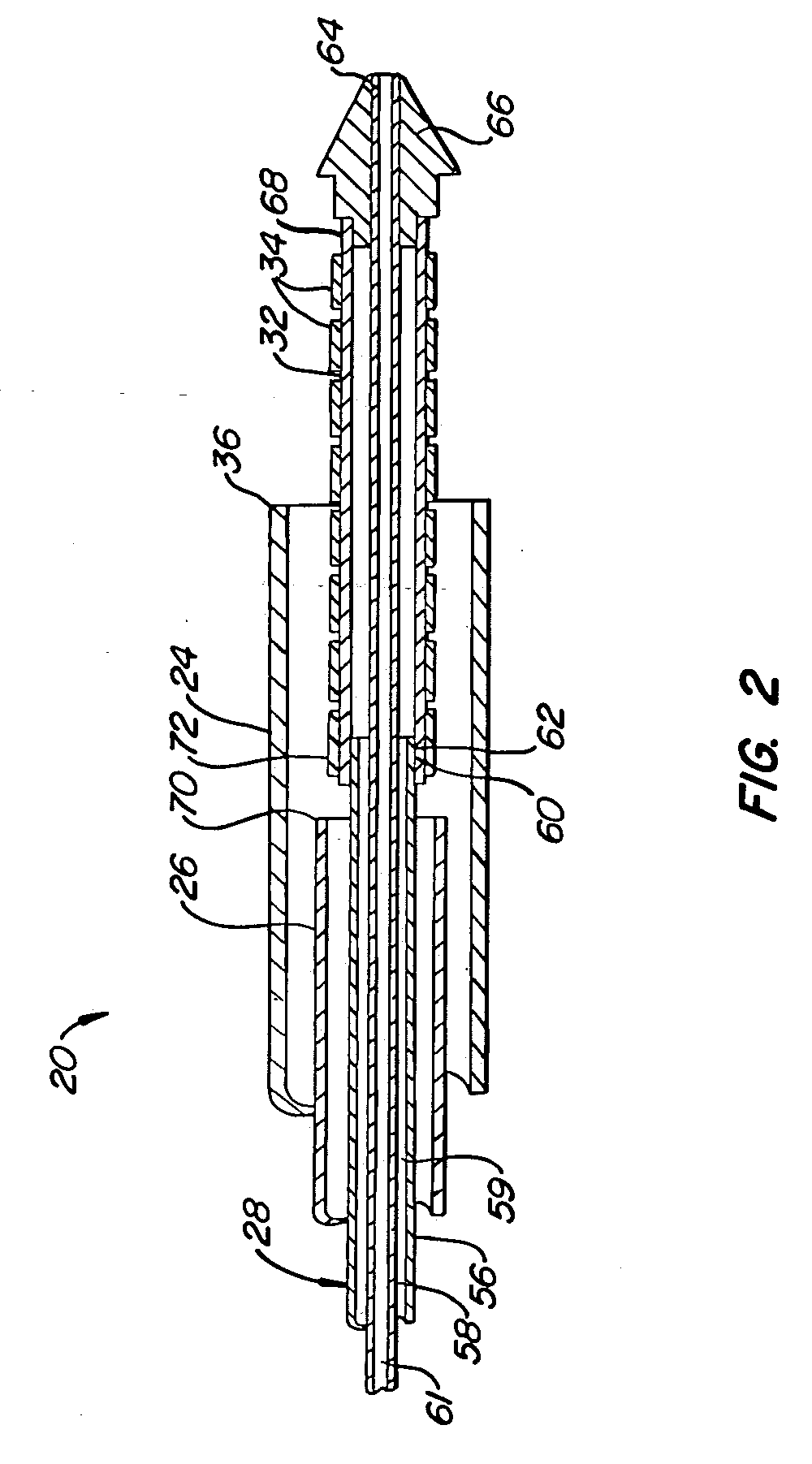
[0034] A preferred embodiment of a stent deployment system according to the invention is illustrated in FIG. 1. Stent deployment system 20 includes a sheath 24, a pusher tube 26 slidably disposed within sheath 24, and a catheter shaft 28 slidably disposed within pusher tube 26. Sheath 24, pusher tube 26 and catheter shaft 28 are all made of a flexible biocompatible material suitable for endovascular placement and positioning along a tortuous path from a peripheral vessel to the coronary arteries. A guidewire 30 is slidably positionable within catheter shaft 28 to facilitate introduction and tracking. An expandable member 32, which preferably is an elastomeric balloon, is mounted to the distal end of catheter shaft 28 and has an unexpanded shape suitable for endovascular positioning, and an expanded shape for deploying a stent within a vascular lumen. A series of tubular stent segments 34 are disposed about the periphery of expandable member 32 and are configured to be expanded by th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com