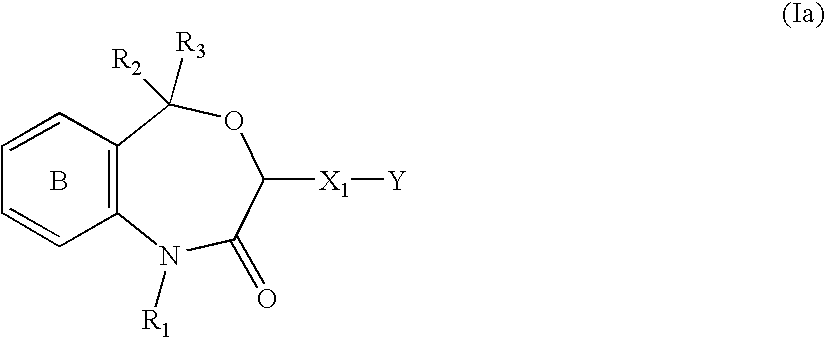
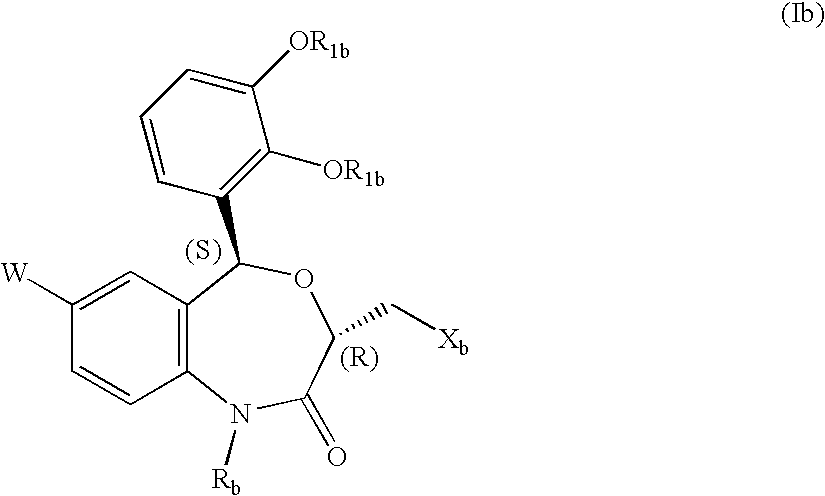
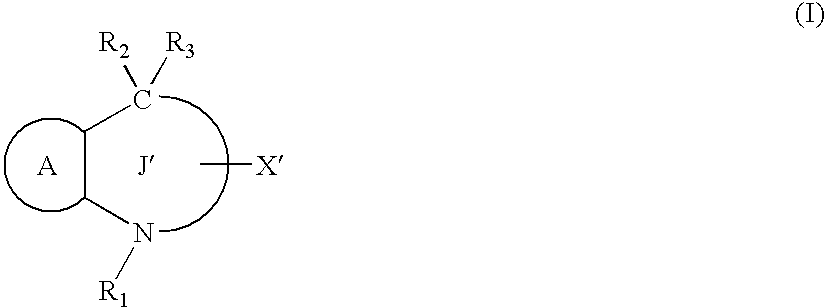Agent for preventing or treating organ functional disorders and organ dysfunction
a functional disorder and organ technology, applied in the field of agents for preventing, treating or suppressing the progress of organ functional disorders and organ dysfunction, can solve the problems of low clinical effect of ubiquinone per se, unclear clinical availability, and general difficulty in ubiquinone migration to organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0539] According to the following composition, a mixture consisting of compound A (175 g), D-mannitol (175 g), corn starch (118.65 g) and crosscarmelose sodium (105 g) is sufficiently mixed using a vertical granulator (FM-VG-10 type, manufactured by Powrex Corporation), and kneaded with an aqueous solution in which hydroxypropyl cellulose (19.25 g) has been dissolved (condition for kneeding: 400 rpm, 10 min). The white-colored kneaded substance is dried using a fluidized drier (FD-3S, manufactured by Powrex Corporation) under the blow temperature of 60° C. for 30 min, and granulated by, using a power mill (model P-3, manufactured by Showa Chemical Machinery Co., Ltd.) and sieving with a 1.5 mmφ punching screen.
[0540] The granule (525.14 g), crosscarmelose sodium (31 g) and magnesium stearate (1.86 g) are added and mixed using a mixer (model TM-15, manufactured by Showa Chemical Machinery Co., Ltd.) for 5 min to give granule for tabletting. The granule is formed, using a tablet form...
preparation example 2
[Preparation of Coating Agent]
[0543] Hydroxypropylmethyl cellulose 2910 (TC-5) (224.4 g) and Macrogol 6000 (45.0 g) were dissolved in purified water (2700 g). To the obtained solution were dispersed titanium oxide (30.0 g) and red iron oxide (0.6 g) to prepare a coating agent.
[Preparation of Tablets]
[0544] Compound A (31.0 g), lactose (3053.5 g) and corn starch (930.0 g) were homogeneously mixed in a fluidized bed granulation dryer (FD-5S, manufactured by Powrex Corporation), and an aqueous solution in which hydroxypropyl cellulose (HPC-L, 139.5 g) had been dissolved was sprayed to perform granulation in the apparatus, then the granule was dried in a fluidized bed granulation dryer.
[0545] The obtained granulated product was crushed by 1.5 mm punching screen using Power Mill grinder (P-3, manufactured by Showa Chemical Machinery Co., Ltd.) to give sized powder.
[0546] To the obtained sized powder (3430 g) were added carmellose calcium (384 g) and magnesium stearate (25.6 g), and ...
preparation example 3
[Preparation of Coating Agent]
[0550] Hydroxypropylmethyl cellulose 2910 (TC-5, 224.4 g) and Macrogol 6000 (45.0 g) were dissolved in purified water (2700 g). To the obtained solution were dispersed titanium oxide (30.0 g) and red iron oxide (0.6 g) to prepare a coating agent.
[Preparation of Tablets]
[0551] Compound A (310.0 g), lactose (2774.5 g) and corn starch (930.0 g) were homogeneously mixed in a fluidized bed granulation dryer (FD-5S, manufactured by Powrex Corporation), and an aqueous solution in which hydroxypropyl cellulose (HPC-L, 139.5 g) had been dissolved was sprayed to perform granulation in the apparatus, then the granule was dried in a fluidized bed granulation dryer.
[0552] The obtained granulated product was crushed by 1.5 mmφ punching screen using Power Mill grinder (P-3, manufactured by Showa Chemical Machinery Co., Ltd.) to give sized powder.
[0553] To the obtained sized powder (3430 g) were added carmellose calcium (384 g) and magnesium stearate (25.6 g), and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| mean particle diameter | aaaaa | aaaaa |
| mean particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com