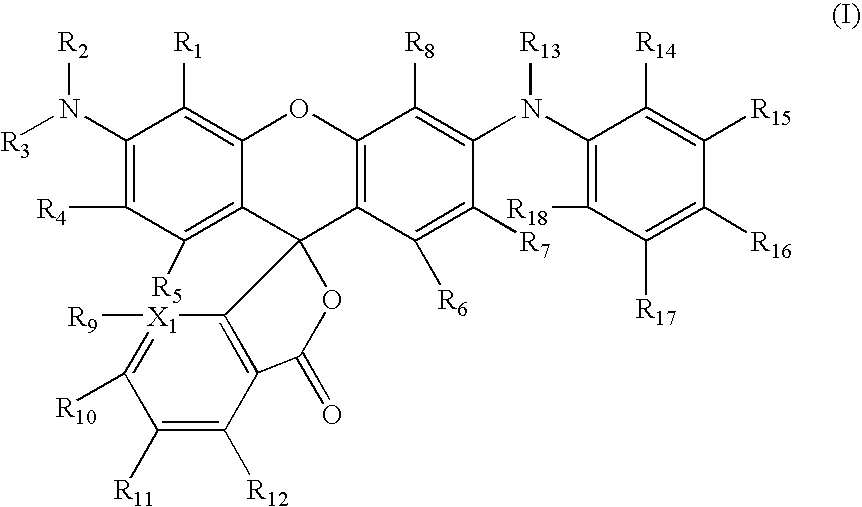
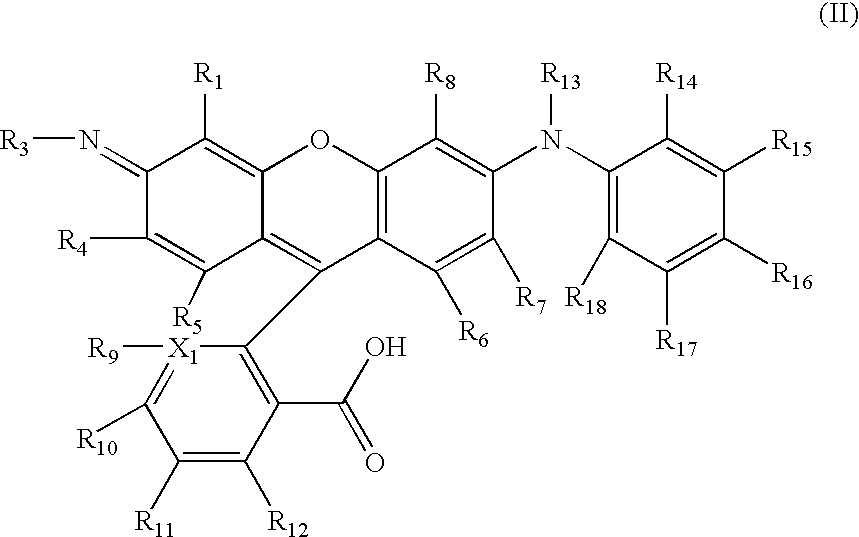
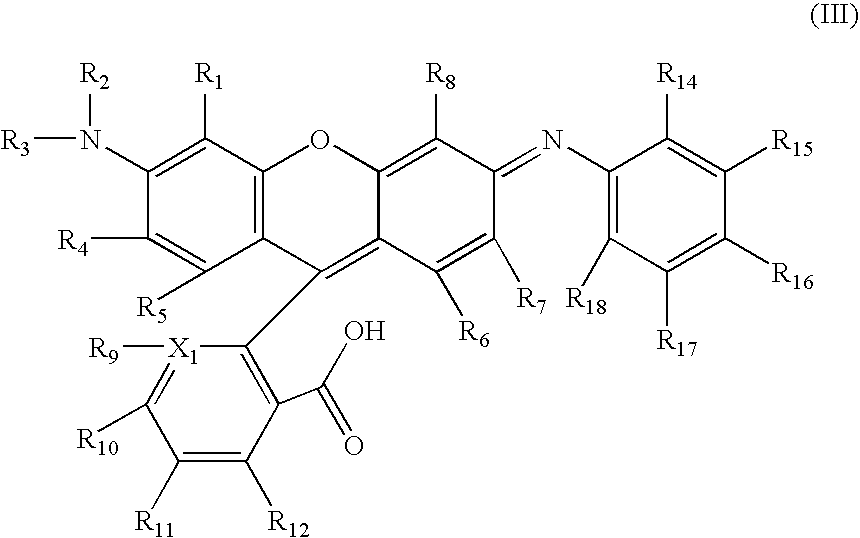
Novel rhodamine dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0072] The invention will now be described further in detail with respect to specific embodiments by way of examples, it being understood that these are intended to be illustrative only and the invention is not limited to the materials, amounts, procedures and process parameters, etc. recited therein. All parts and percentages recited are by weight unless otherwise specified.
example i
Synthesis of N-acetyl-N-octylaniline
[0073] 1-octyl bromide (39 mL, 224 mmol, 1.12 eq) was added dropwise to a mixture of acetanilide (27 g, 200 mmol, 1 eq.) in dimethylsulfoxide (130 mL), containing potassium hydroxide pellets (18.87 g, 300 mmol, 1.5 eq.), at room temperature. After all the 1-octyl bromide was added the reaction mixture was heated to 50-55° C. for 1.5 hours. The reaction mixture was cooled and poured into water (1 L), stirred for 45 minutes and extracted with hexanes (3×400 mL). The hexane extracts were combined, dried over sodium sulfate and evaporated to give 48.4 g (196 mmol, 98%) of colorless oil. The product was identified by NMR spectroscopy and mass spectrometry and was used without further purification.
Synthesis of N-octylaniline
[0074] To N-acetyl-N-octylaniline (48.4 g, 196 mmol) was added 4N hydrochloric acid (100 mL) and the mixture heated to 100-110° C. and stirred at this temperature for 4 days. The reaction mixture was cooled to ambient temperature,...
example ii
Synthesis of Dye I
[0082] A mixture of 3′-chloro-6′-tetrahydroquinolinofluoran (100 mg, 0.2 mmol), decylamine (100 mg, 0.6 mmoles), and zinc chloride (100 mg, 0.7 mmol) in sulfolane (3 mL) was held at 150° C. for 3 hours. The reaction mixture was quenched into 10 ml of water. The solid was filtered off, washed with water and dried. The product was purified by silica gel chromatography using 2% methanol in methylene chloride to yield Dye I, 3′N-decylamino-6-tetrahydroquinoline fluoran as an off-white solid (42 mg, 0.07 mmol, 35%). The product was identified by NMR spectroscopy and mass spectrometry, m.p. 124° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com