Differentiation of non-insulin producing cells into insulin producing cells by GLP-1 or exendin-4 and uses thereof
a technology of exendin-4 and non-insulin producing cells, which is applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of beta cell absence, diabetes mellitus, and functional impairmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
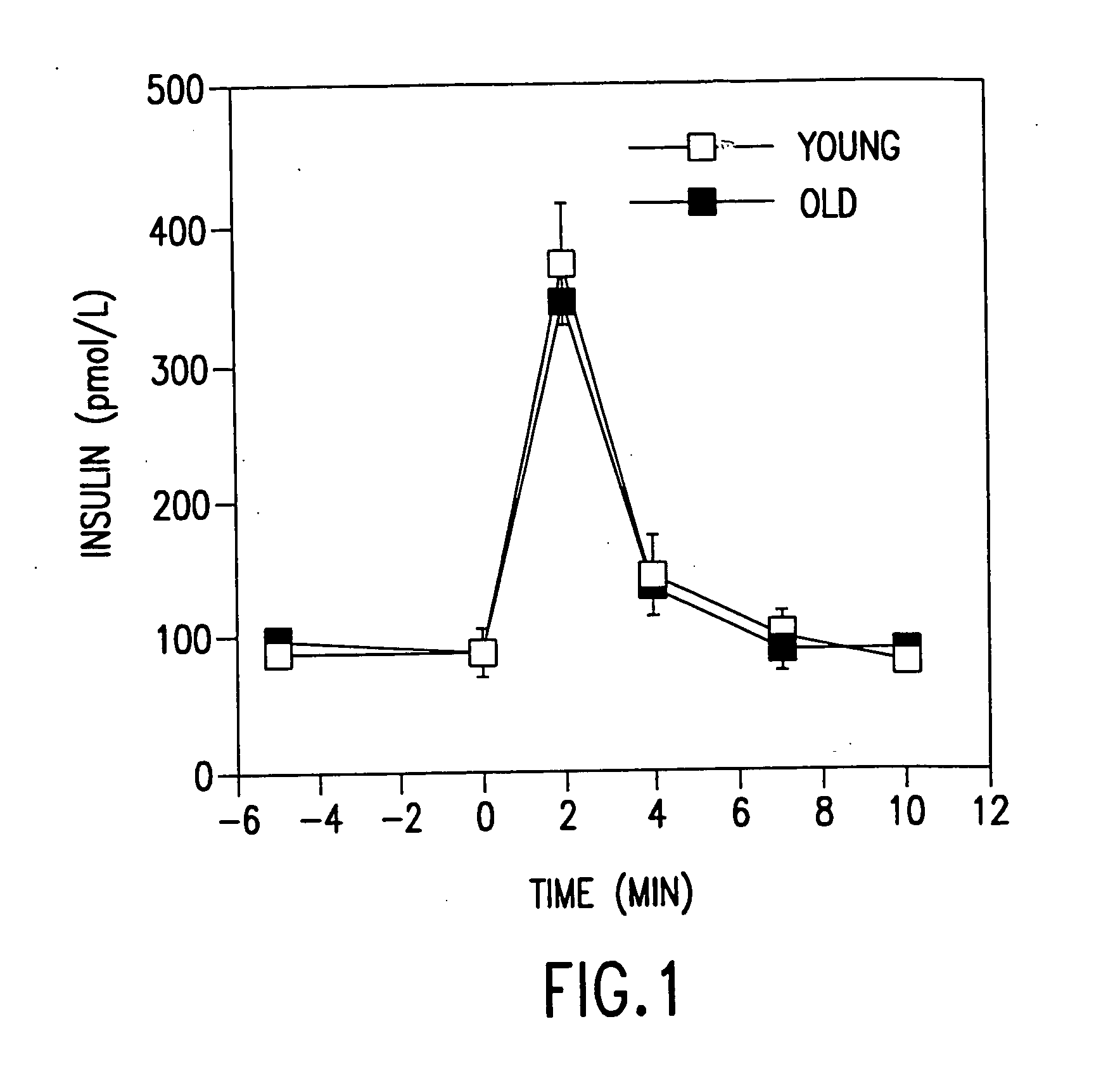
[0080] As GLP-1 in cultured insulinoma cells is known to impact positively on insulin secretion, insulin synthesis and insulin messenger RNA, GLP-1's effects on aging Wistar rats were evaluated.
[0081] Materials. GLP-1 and exendin [9-39] (Ex), a peptide receptor antagonist of GLP-1, were purchased from Bachem (King of Prussia, Pa.). Chemical reagents were from Sigma (St Louis, Mo.), unless otherwise stated.
[0082] Animals. Three month (young) and 22 month (old) old Wistar rats from the Wistar colony in the NIA (Baltimore, Md.) were used. They had been maintained on rat chow and fed ad libitum. All our aged rats are the offspring of ten founder families maintained at the NIA.
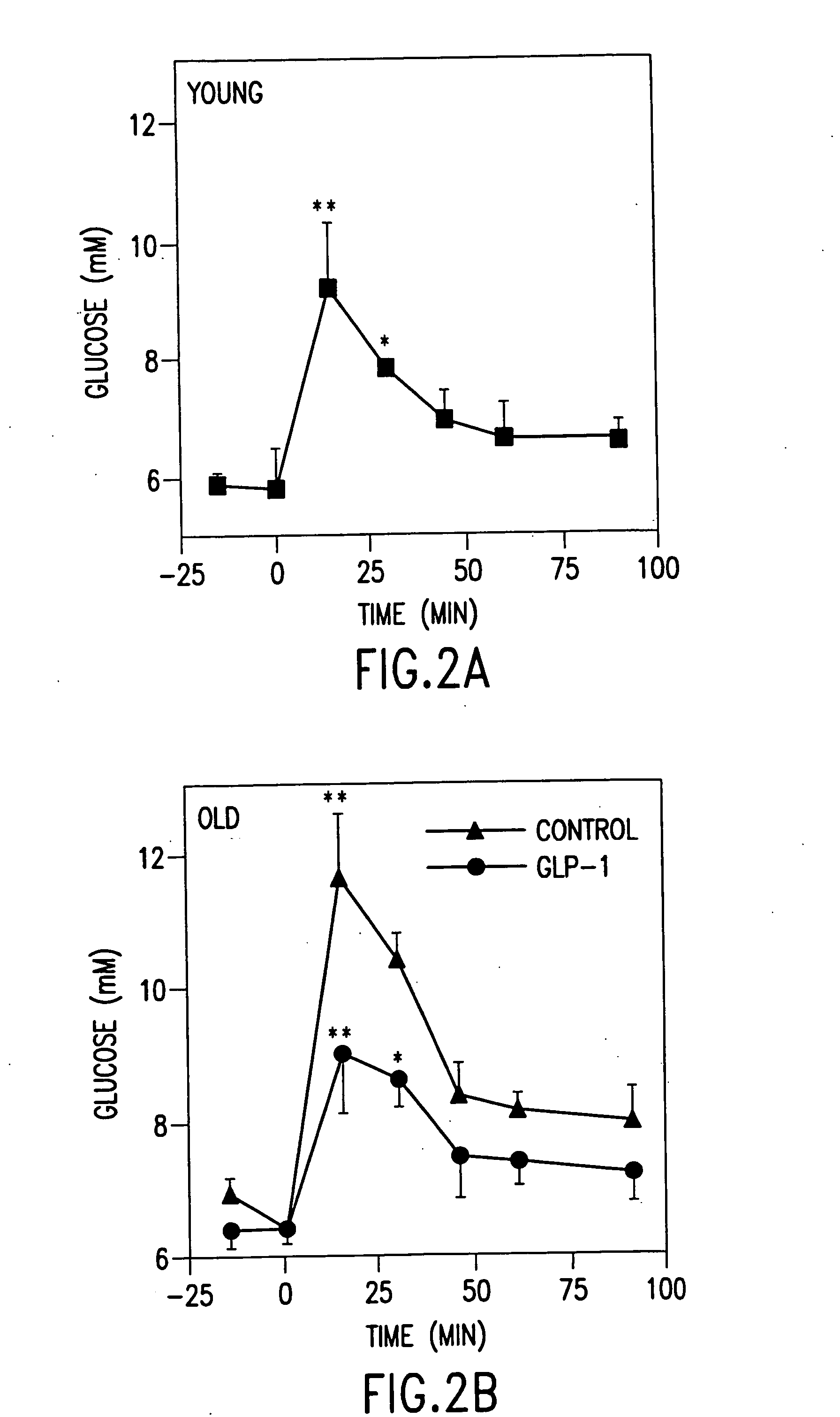
[0083] Protocols. To insure that old animals were capable of responding to GLP-1, we carried out an acute experiment with an intravenous bolus of GLP-1. Six old (22 months) and 6 young (3 months) Wistar rats were fasted overnight. Following anesthesia with 50 mg / kg pentobarbital, a catheter was placed in the fem...
example 2
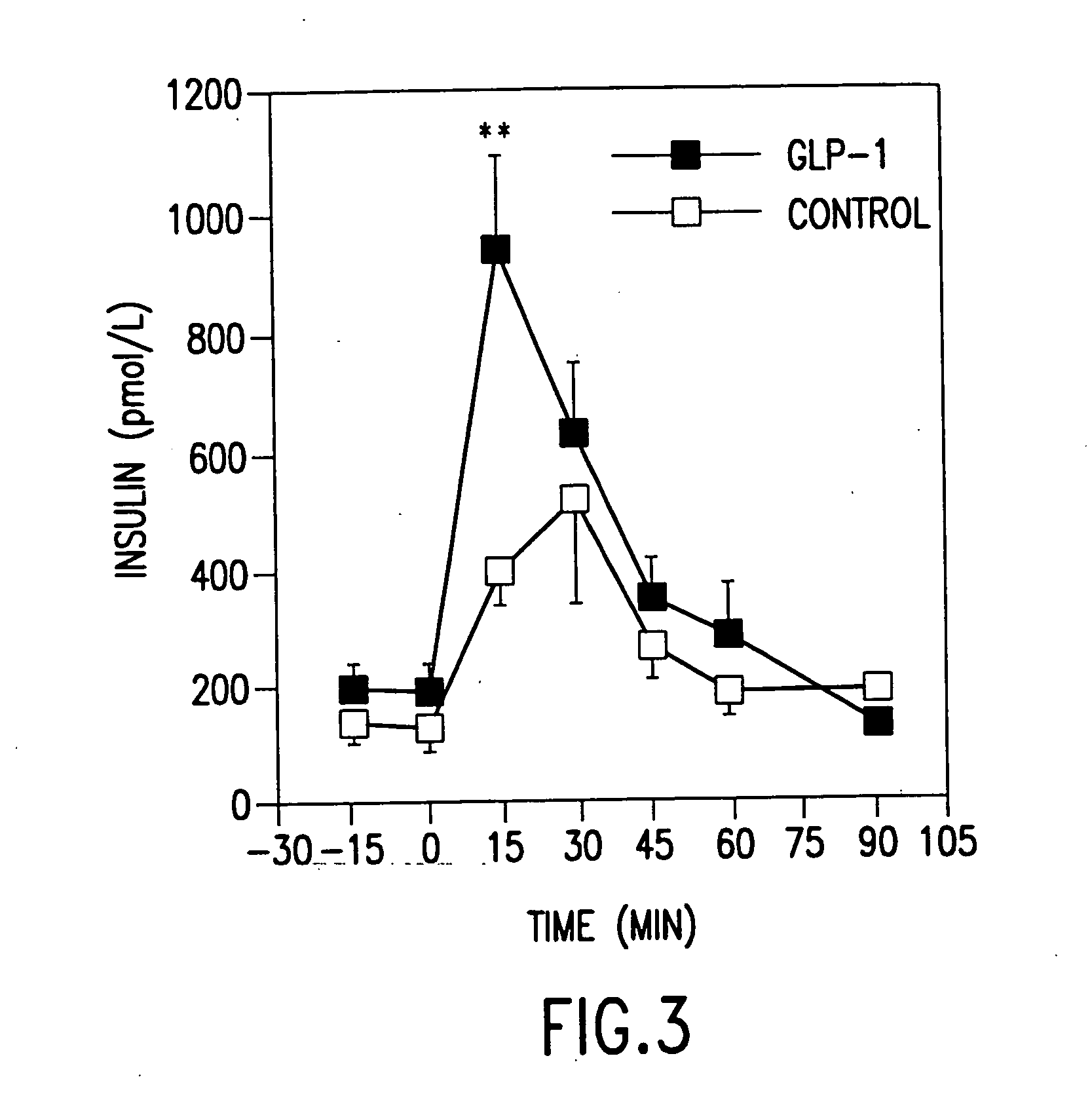
[0105] Exendin-4 is a peptide produced in the salivary glands of the Gila Monster lizard. In the present example, we report that in Wistar rats, bred in the National Institute of Aging (NIA), it was a far more potent insulinotropic agent in several ways than is GLP-1. We further report that exendin-4 leads to sustained improvement of diabetic control in a rodent model of type 2 diabetes.
[0106] Materials. Exendin-4 and GLP-1 were purchased from Bachem (King of Prussia, Pa.). Chemical reagents were from Sigma (St Louis, Mo.), unless otherwise stated.
[0107] Animals. Four month old Wistar rats from the Wistar colony in the NIA (Baltimore, Md.) were used for the acute experiments of the effects of exendin-4 and GLP-1 (see Example 1). They had been maintained on standard lab chow and fed ad libitum. For the long-term experiments, diabetic mice (C57BLKS / J-Leprdb / Leprdb) lacking the leptin receptor, and their non-diabetic littermates were purchased at 4 weeks of age from Jackson Laborator...
example 3
[0126] Using the protocol of Example 1, GLP-1 was administered by continuous infusion for one to five days to young and old rats, whereas control rats received comparable saline infusions. Exendin-4, in contrast, was administered intraperitoneally one time daily for five days according to the protocol of Example 2.
[0127] Approximately 20% of the cells in the GLP-1 treated pancreata were positive for PCNA at five days. At the same time point, there were proliferating cells in the islet. In addition, there were proliferating cells lining the ducts and, more surprisingly, in the acinar tissue, an area generally considered to be devoid of stem cells. Also surprisingly, a number of insulin positive cells were found outside the islets among the acinar tissue, where insulin positive cells are not expected.
[0128] These results show that continuous infusion with GLP-1 or repeated intraperitoneal injection with Exendin-4 for at least two days results in an increase in total number of insuli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com