Method for administration of therapeutic gases
a technology for administering therapeutic gases and gases, which is applied in the direction of mechanical equipment, valves, operating means/releasing devices, etc., can solve the problems of not being able to administer therapeutic gases for short periods of time for specific medical purposes, significant problems in terms of handling and use, and not being able to use self-administration, etc., to achieve the effect of easing patient pain and anxiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Administration of Nitrous Oxide / Oxygen Mixture to Healthy Volunteers
[0385] Initially, pilot studies were conducted on 5 healthy volunteers having medical experience of dealing with patients with AF-ICDs. The volunteers of different ages and weights were selected. The volunteers had experience with N2O ranging from none to some and from long ago to recent. The volunteers were witnessed and interviewed about their experience. They were asked to visualize their worse historical pain. The volunteers were also asked to provide an input from their patients on how an AF-ICD shock feels, including information about the anxiety generated by memory of the prior shocks before the next shock is initiated.
[0386] The volunteers, while being videotaped, were then asked to breathe 65% N2O / 35% O2 mixture (expressed in molar percents) for 4 minutes while periodically pressing an activator button to simulate pressing of the shock timer button on an AF-ICD, and to determine the ability to press the t...
example 2
Administration of Nitrous Oxide / Oxygen Mixture to Actual Patient Volunteers Having an Implanted AF-ICD
[0387] Eleven patients having an existing implanted AF-ICD for atrial fibrillation participated in a study that included self-administration of nitrous oxide / oxygen mixture prior to administration of an AF-ICD shock. The patients were asked to breathe 65% N2O / 35% O2 mixture for 4 minutes. The nitrous oxide / oxygen mixture was self-administered using the prior art N2O / O2 mixing system described in Example 1 using an inspiration-activated demand valve under observation. The patients were asked to periodically press an activator button, which was not connected to the AF-ICD, to evaluate their responsiveness to commands and ability to self-initiate an AF-ICD shock timer during the short period of nitrous oxide / oxygen administration. When a patient indicated that he / she was ready to actually self-activate the AF-ICD timer to administer a shock and pressed a button to simulate the self-ac...
example 3
Prescription of System 100 and Cassette 200 for Patients Having Implanted AF-ICD
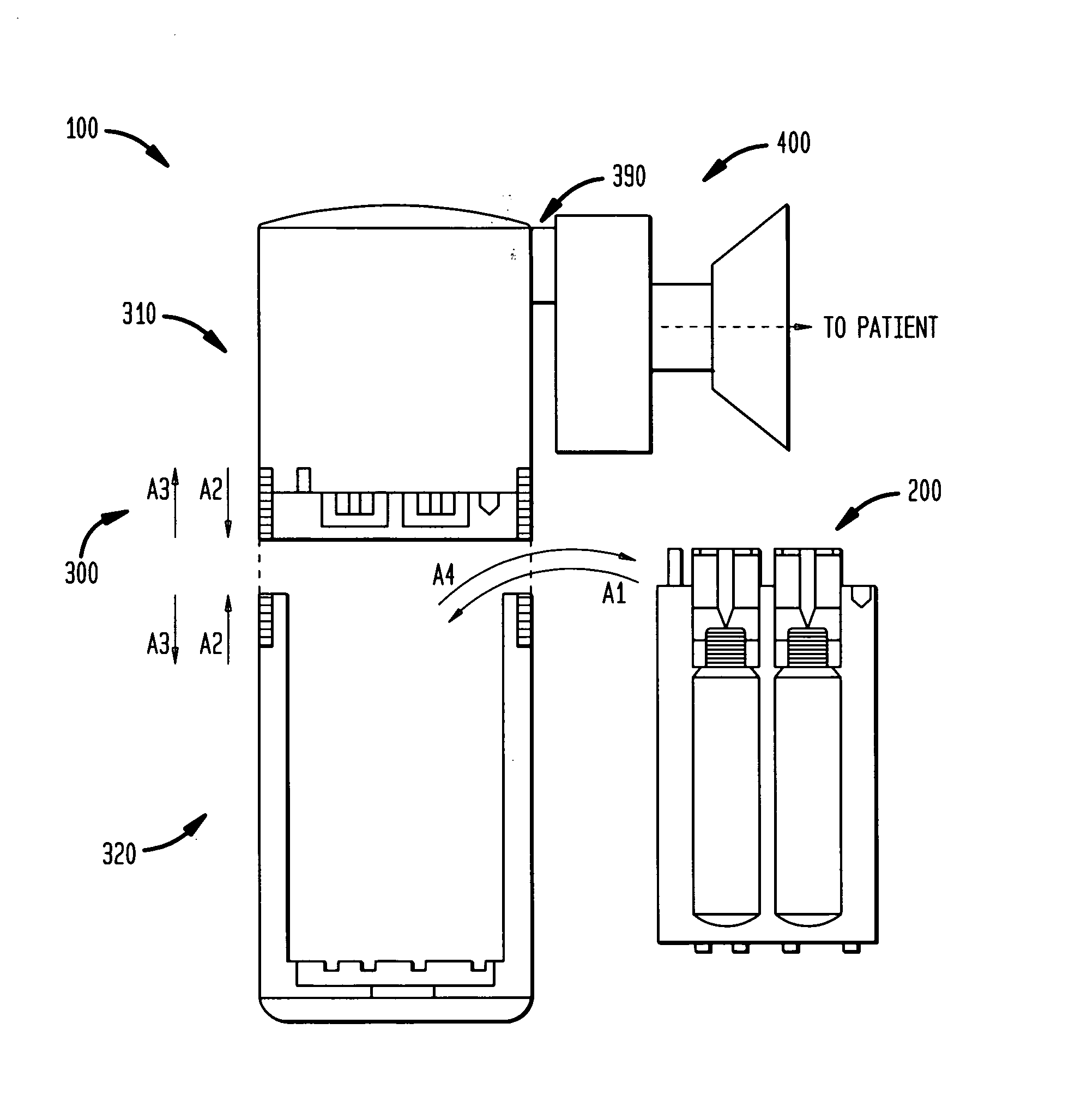
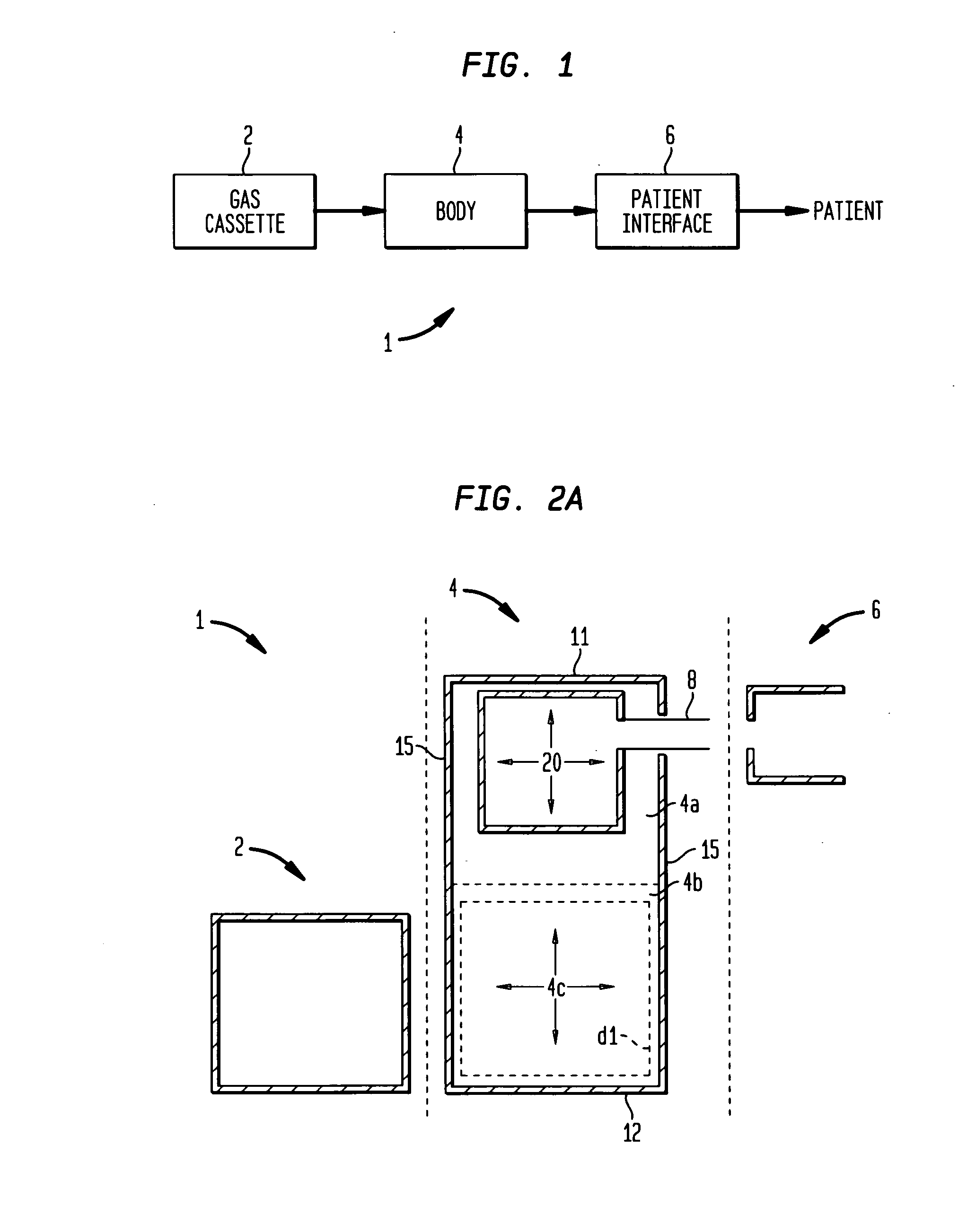
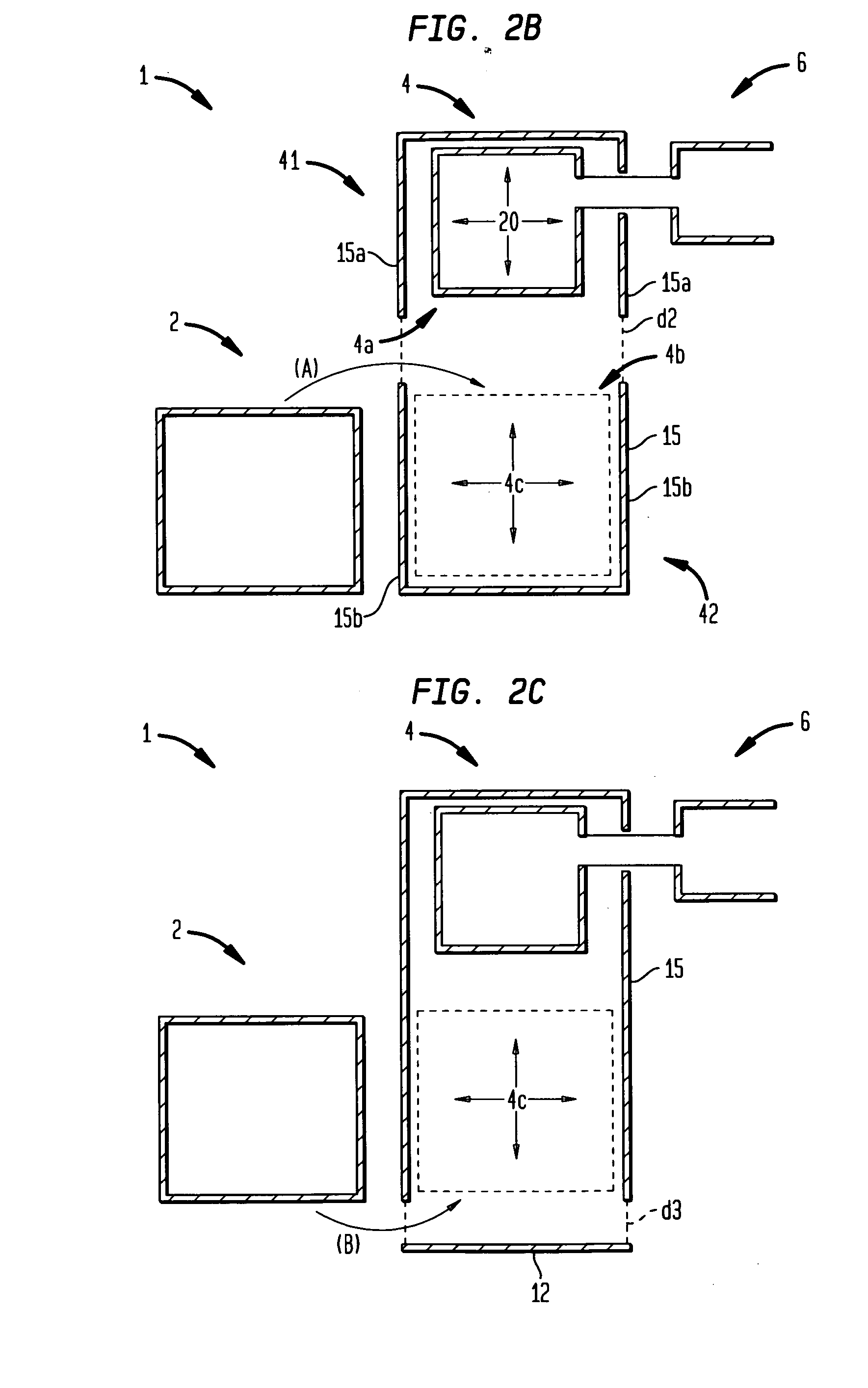
[0389] A physician wishes to prescribe the 65% N2O / 35% O2 mixture to patient X who has an implanted AF-ICD. The prescribing physician is cardiologist K. The prescription is intended for self-administration of the nitrous oxide / oxygen mixture to relieve the pain and anxiety associated with self-initiation of the patient X's AF-ICD in outpatient setting. In practice sessions with the patient X, the cardiologist K had determined that approximately 4 minutes of gas administration are sufficient to produce the desired analgesia and anxiolysis for the patient X.
[0390] The patient X first obtains the body 300 (as well as the patient interface assembly 400). The electrophysiologist or cardiologist K may directly provide the patient X with the body 300 and the assembly 400. Alternatively, a pharmacist or a manufacturer provides the body 300 to the patient X on the basis of the prescription from the electrophysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com