Immuno-gold lateral flow assay
a technology of lateral flow and immunoglobulin, which is applied in the field of immunoglobulin lateral flow assay and chromatographic flow binding assay, can solve the problems of low detection efficiency, low detection efficiency, and low detection efficiency of ligands, and achieves rapid high sensitivity, high sensitivity and specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
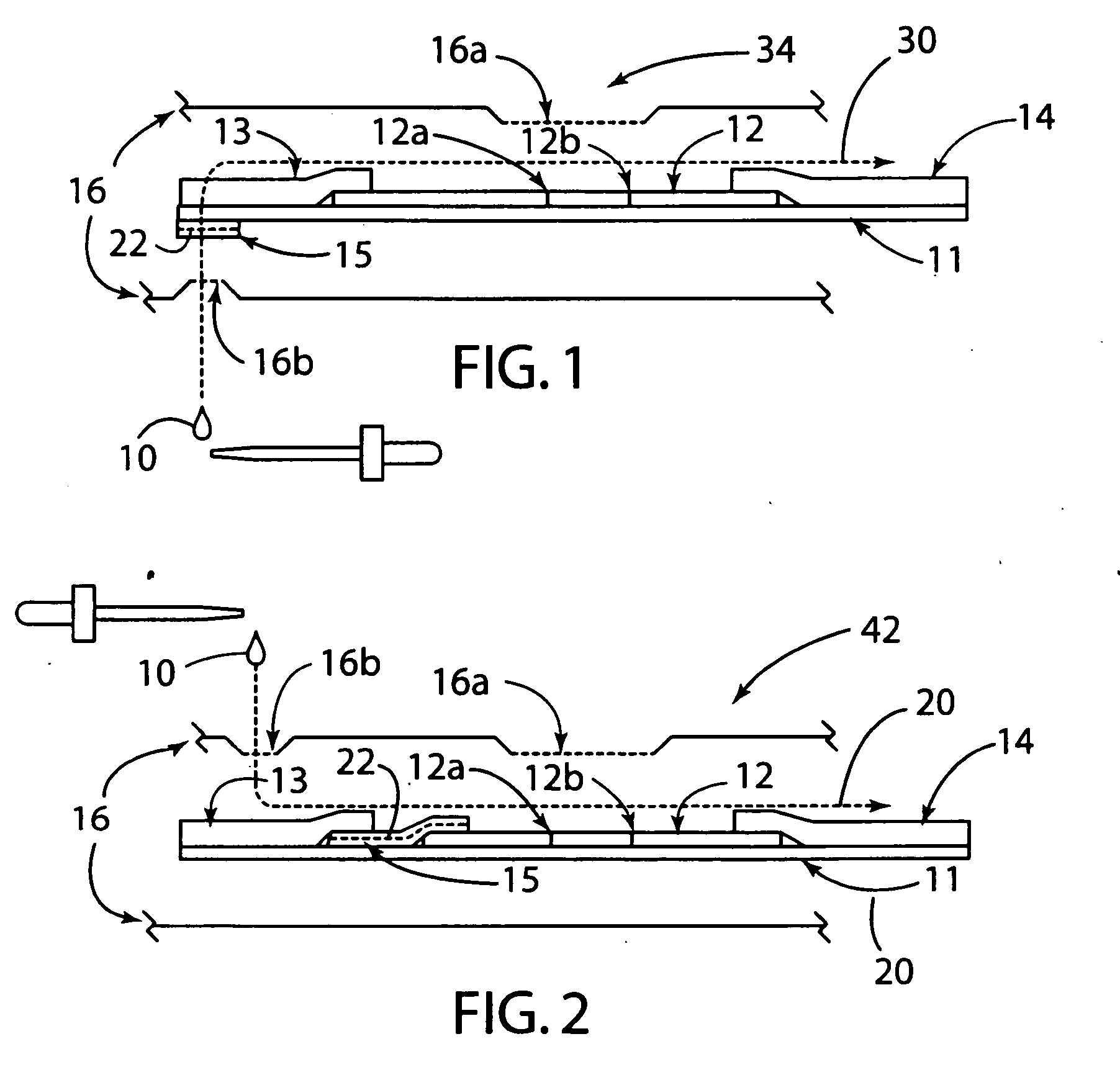
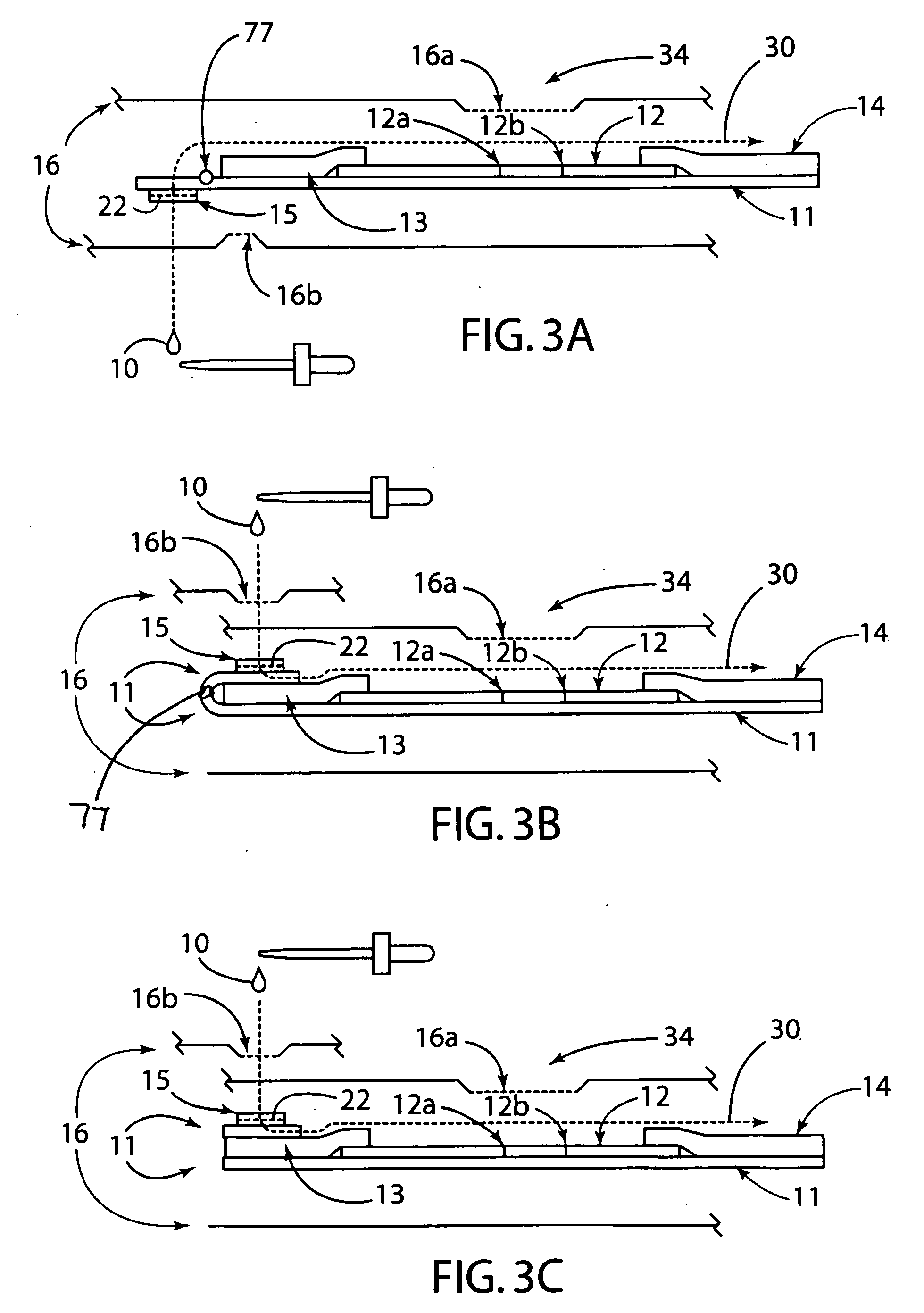
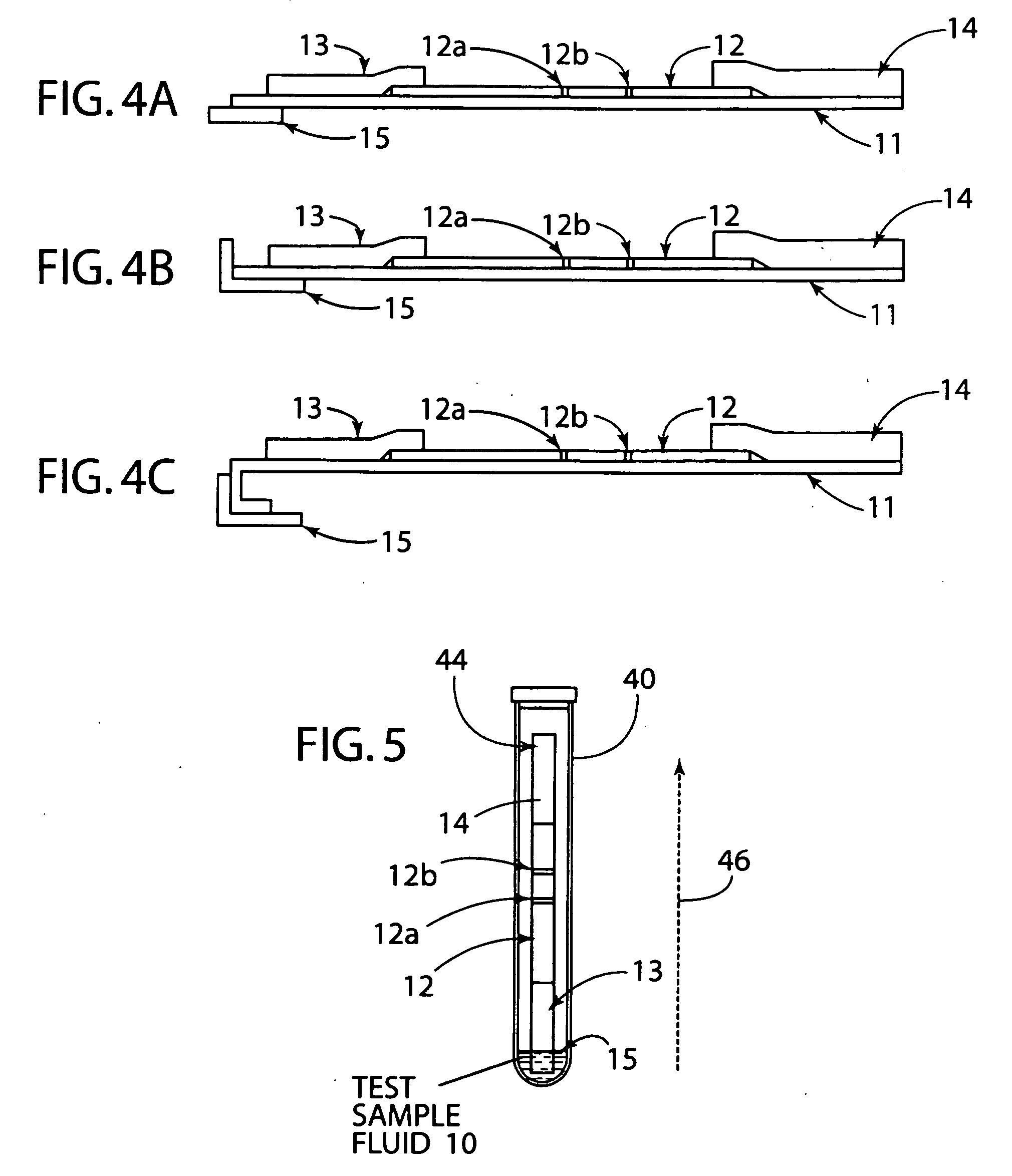
[0033] The present invention generally relates to a rapid, high sensitivity chromatographic assay for detecting low levels of ligands in bio-fluids, environment, plant and tissue culture extracts, using a minimal number of procedural steps even when used by untrained persons. The present invention encompasses diagnostic kits that may contain a chromatographic specific binding assay system, and preferably an immunochromatographic specific binding assay system. Furthermore, the system and apparatus, because of its accuracy and simple method steps, make it appropriate for field use such as a home, clinic, point of care setting, or doctor's office. Test results may be visually read or read by an instrument known in the art and readily available to give either a semi-qualitative (e.g., a positive / negative result) or a quantitative result. In use, the present invention is simple to use and requires a minimum degree of user skill and involvement.
[0034] To assist in understanding the prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com