In vivo cell surface engineering
a cell surface and in vivo technology, applied in the field of in vivo cell surface engineering, can solve the problems of less than satisfactory clinical response, inability to achieve satisfactory clinical response, and inability to achieve satisfactory clinical response, and achieve the effect of reducing the size of the tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
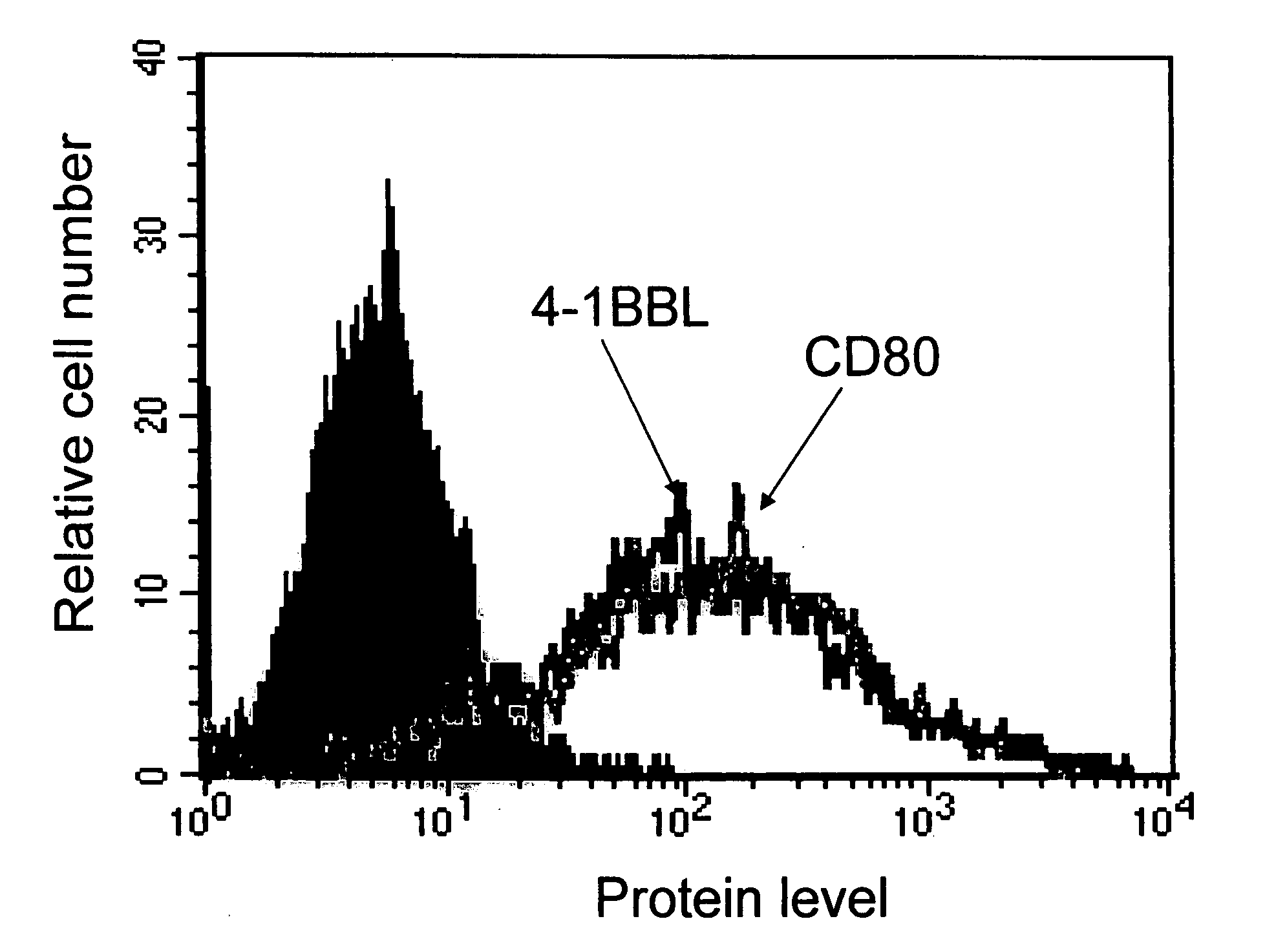
[0143] Results herein demonstrate that (1) tumor and primary cells can effectively be biotinylated under physiological conditions and biotin persists on the cell surface for weeks; (2) biotinylated cells can be engineered with several fusion proteins that then persist on the cell surface in vivo for extended periods of time (days to weeks); (3) subcutaneous injection of live LLC or A20 tumor cells causes solid tumors in syngeneic mice. The data show that tumors can be engineered in vivo for the display of CD80.
[0144] A series of studies was performed using EZ-Link™ Sulfo-NHS-LC-Biotin derivative from Pierce Biotechnology, Inc. (Rockford, Ill.) to establish biotinylation conditions that do not compromise the viability and functions of the cells. Biotinylation in the range of 5-50 μM satisfied these criteria and provided an effective platform for the optimum display of exogenous proteins. Yolcu et al., 2002, Immunity, 17:795-808.
[0145] To assess the kin...
example 2
[0166] This Example describes the engineering of tumor surfaces in vivo for effective and durable display of a combination of two or more immune co-stimulatory polypeptides, such as two or more of LIGHT, CD80, 4-1BBL, CD40L, OX40L, PD-L1 and GL50 polypeptides.
[0167] An effectivce biotin dose for the biotinylation of tumor cells in vivo and the kinetics of biotin persistence on the surface of such tumor cells can be determined. An effective level of biotin conjugation in vivo can be defined as (i) biotinylation of >50% of tumor cells; (ii) biotin persistence on the surface of at least 50% of the tumor cells initially positive for biotin 5 days post-biotinylation, wherein the mean fluorescence intensity of the biotin positive cells is >70; and (iii) no detectable toxicity for either the tumor or the patient.
[0168] Doses of biotin that satisfy these criteria should permit display of >100,000 immune co-stimulatory fusion protein molecules per tumor cell immediately a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| soluble | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com