Screening For Solid Forms By Ultrasound Crystallization And Cocrystallization Using Ultrasound
a technology of cocrystallization and ultrasound, which is applied in the direction of glucose production, separation processes, instruments, etc., can solve the problems of reducing or eliminating the utility or beneficial purpose, reducing or eliminating the utility of the effect, and pharmaceutical industry spending a great deal of time, effort and expense on the identification of particular compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
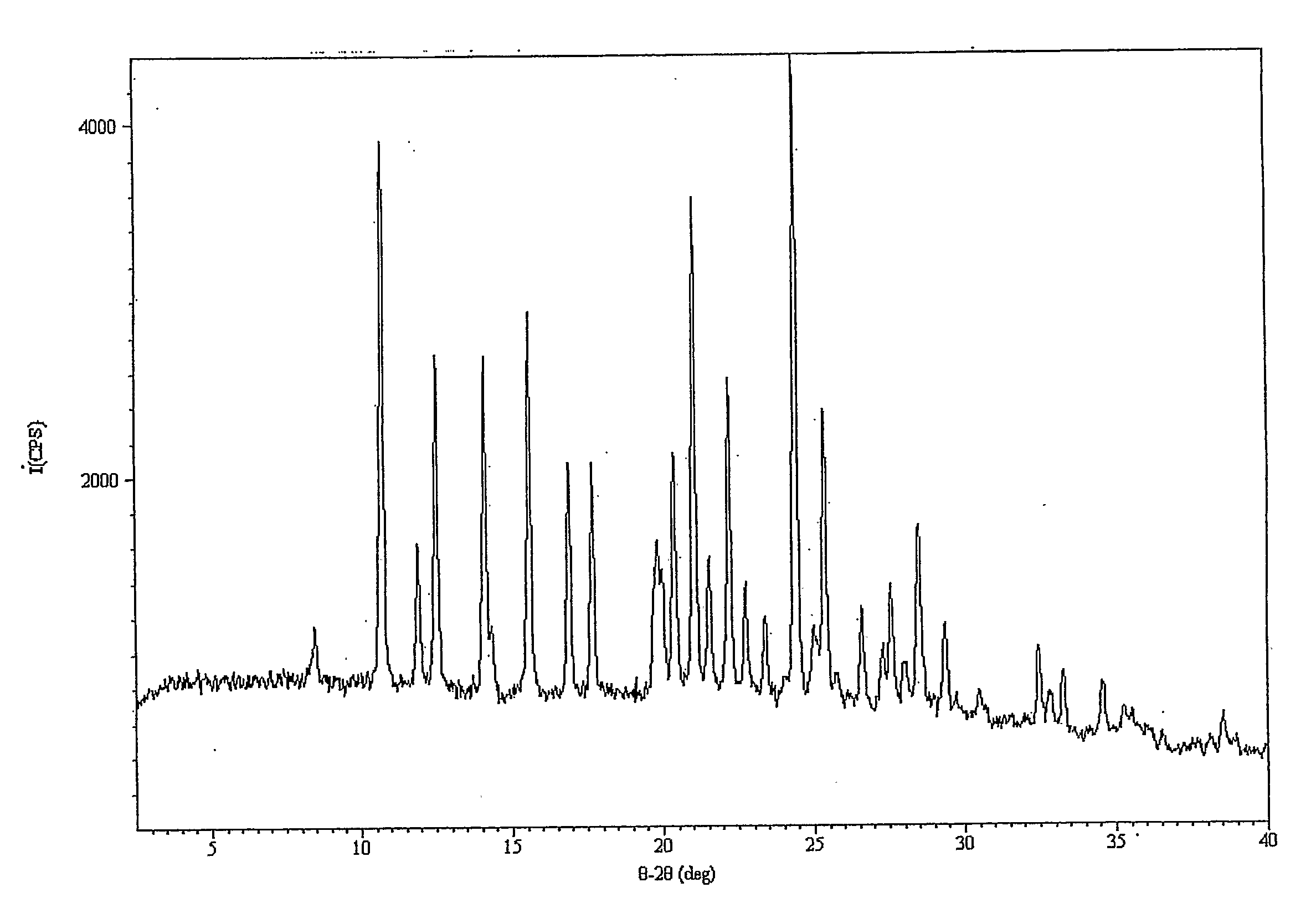
[0324] In this Example, a new solid form of sulfathiazole was prepared from solution in acetonitrile using sonication. This form was not seen without sonication under the same conditions. Form II of sulfathiazole was generated without sonication.
[0325] A saturated solution of sulfathiazole in acetonitrile at 45° C. was prepared, filtered hot and split between two pre-heated 1-dram vials (about 1 ml each). The samples were left to slowly cool to room temperature. One sample was then nucleated by ultrasound treatment using 5 pulses of one second each at 20 kHz, amplitude control set at 40, using a Cole Palmer ultrasonic processor CP130 fitted with a 6 mm tip stainless steel probe, while the other sample was left undisturbed (unsonicated). Both samples were then left to evaporate slowly to dryness. XRPD patterns of the sonicated and unsonicated samples showed that the sonicated sample gave an unknown pattern (FIG. 1) whereas the unsonicated sample yielded the known Form II of sulfathi...
example 2
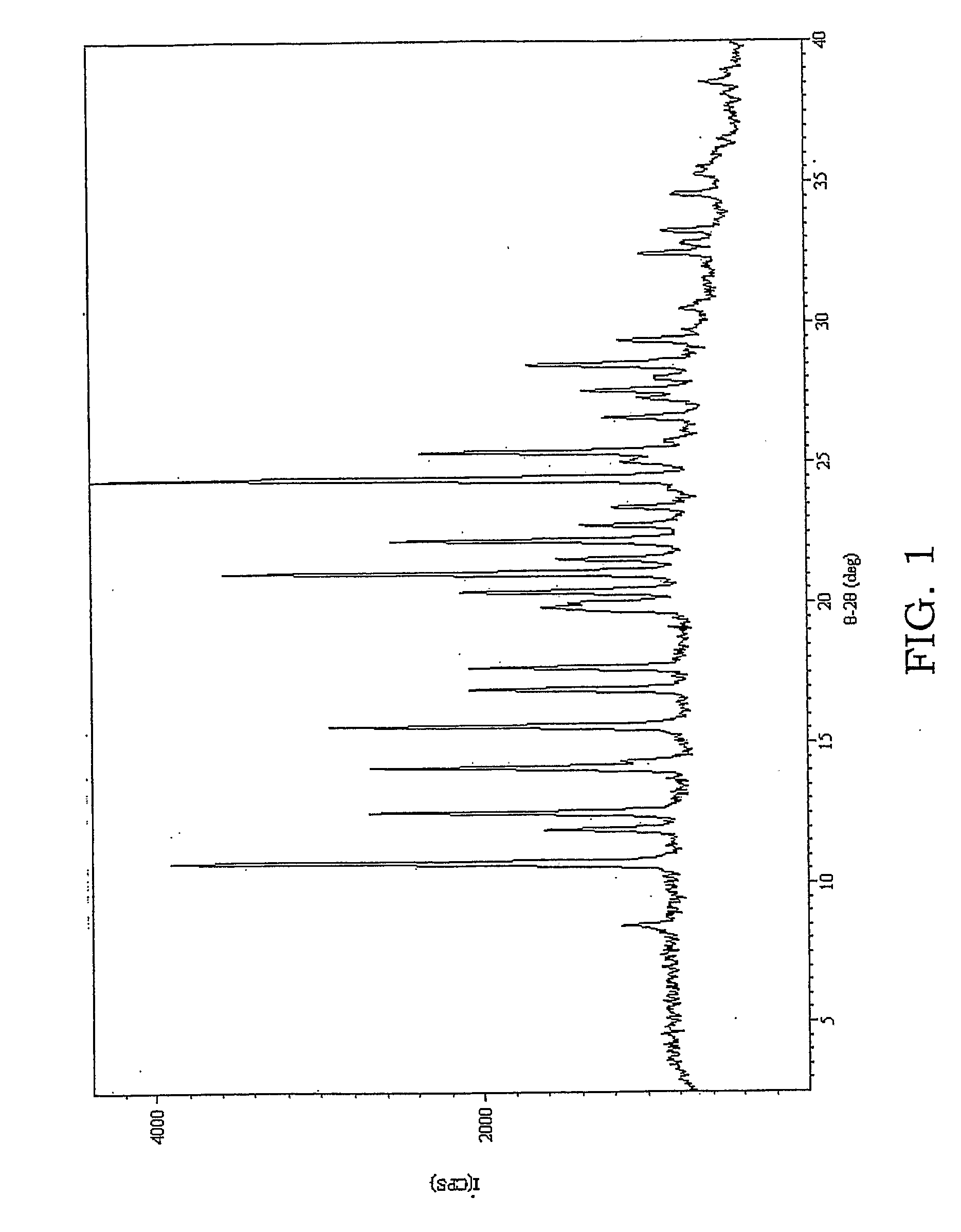
[0326] In this Example, an unusual solid form of an acetone solvate of sulfathiazole was prepared. Without sonication, this form was only seen in a mixture with the known form of an acetone solvate of sulfathiazole.
[0327] A saturated solution of sulfathiazole in acetone at 45° C. was prepared, filtered hot and split between two pre-heated 1-dram vials (about 1 ml each). The samples were left to slowly cool to room temperature. One sample was then nucleated by ultrasound treatment using 5 pulses of one second each at 20 kHz, amplitude control set at 40, using a Cole Palmer ultrasonic processor CP130 fitted with a 6 mm tip stainless steel probe, while the other sample was left undisturbed (unsonicated). Both samples were then left to evaporate slowly to dryness. XRPD patterns of the sonicated and unsonicated patterns showed that the sonicated sample gave an unknown pattern (FIG. 3) whereas the unsonicated sample yielded a mixture of this same form with the known acetone solvate of su...
example 3
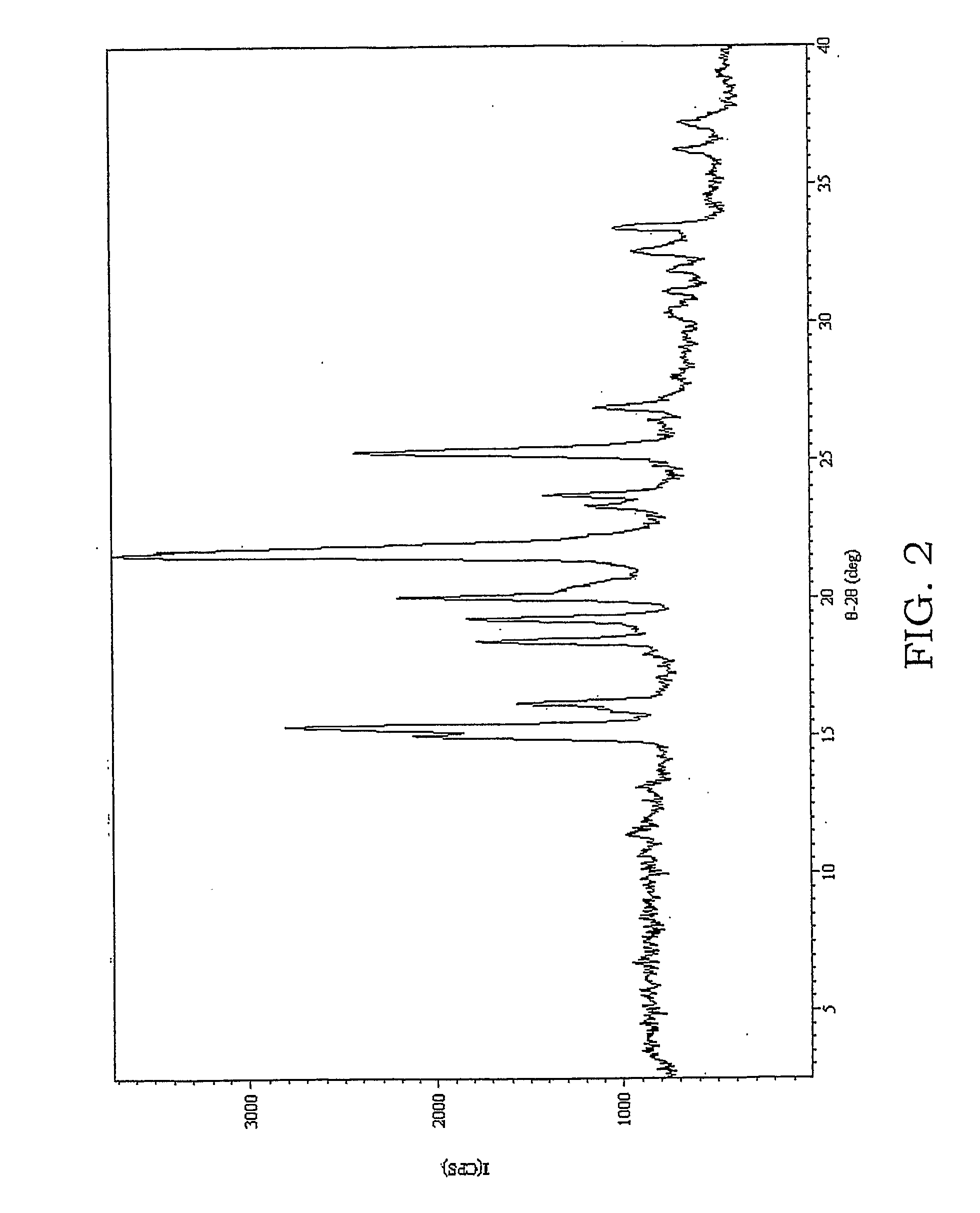
[0328] In this Example, a new DMSO solvate of carbamazepine was prepared.
[0329] Three 100 μl samples of a saturated solution of carbamazepine in DMSO at 50° C. were placed in 3 pre-heated HPLC vials. The samples were allowed to cool to ambient temperature. One sample was nucleated by ultrasound treatment (5 one-second pulses, 20 kHz, amplitude control set at 40, using the Cole Palmer ultrasonic processor with a 3 mm tip stainless steel probe), while another was stirred using a stir bar and the other was left undisturbed (unsonicated). While the sonicated sample yielded a new DMSO solvate, the other samples remained as solutions indefinitely. The XRPD pattern of the new DMSO solvate is shown in FIG. 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com