Solid dressing for treating wounded tissue
a wound tissue and dressing technology, applied in the direction of drug compositions, extracellular fluid disorders, peptide/protein ingredients, etc., can solve the problems of unsatisfactory bleeding stopper materials and methods available in pre-hospital care (gauze dressings, direct pressure, tourniquets), occurrence of excessive bleeding or fatal hemorrhage from an accessible site, and unsatisfactory effects in the past 2000 years
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0103] Backing material (DEXON™) was placed into 2.4×2.4 cm PETG molds. Twenty-five microliters of 2% sucrose was pipetted on top of each of the four corners of the backing material. Once completed the molds were placed in a −80° C. freezer for at least 60 minutes.
[0104] Fibrinogen (Enzyme Research Laboratories™ (ERL) lot 3114) was formulated in 100 mM Sodium Chloride, 1.1 mM Calcium Chloride, 10 mM Tris, 10 mM Sodium Citrate, 1.5% Sucrose, Human Serum Albumin (HSA) at a concentration of 80 mg / g of total protein and 15 mg / g total protein of Tween™ 80 (animal source) (Complete Fibrinogen buffer (CFB)). The fibrinogen concentration was adjusted to 37.5 mg / ml using CFB. The final pH of the fibrinogen was 7.4±0.1. Once prepared the fibrinogen was placed on ice until use.
[0105] Thrombin was formulated in 150 mM Sodium Chloride, 40 mM Calcium Chloride, 10 mM Tris and 100 mM L-Lysine with the addition of HSA at 100 ug / ml (Complete Thrombin Buffer (CTB)). The final pH of the thrombin was ...
example 2
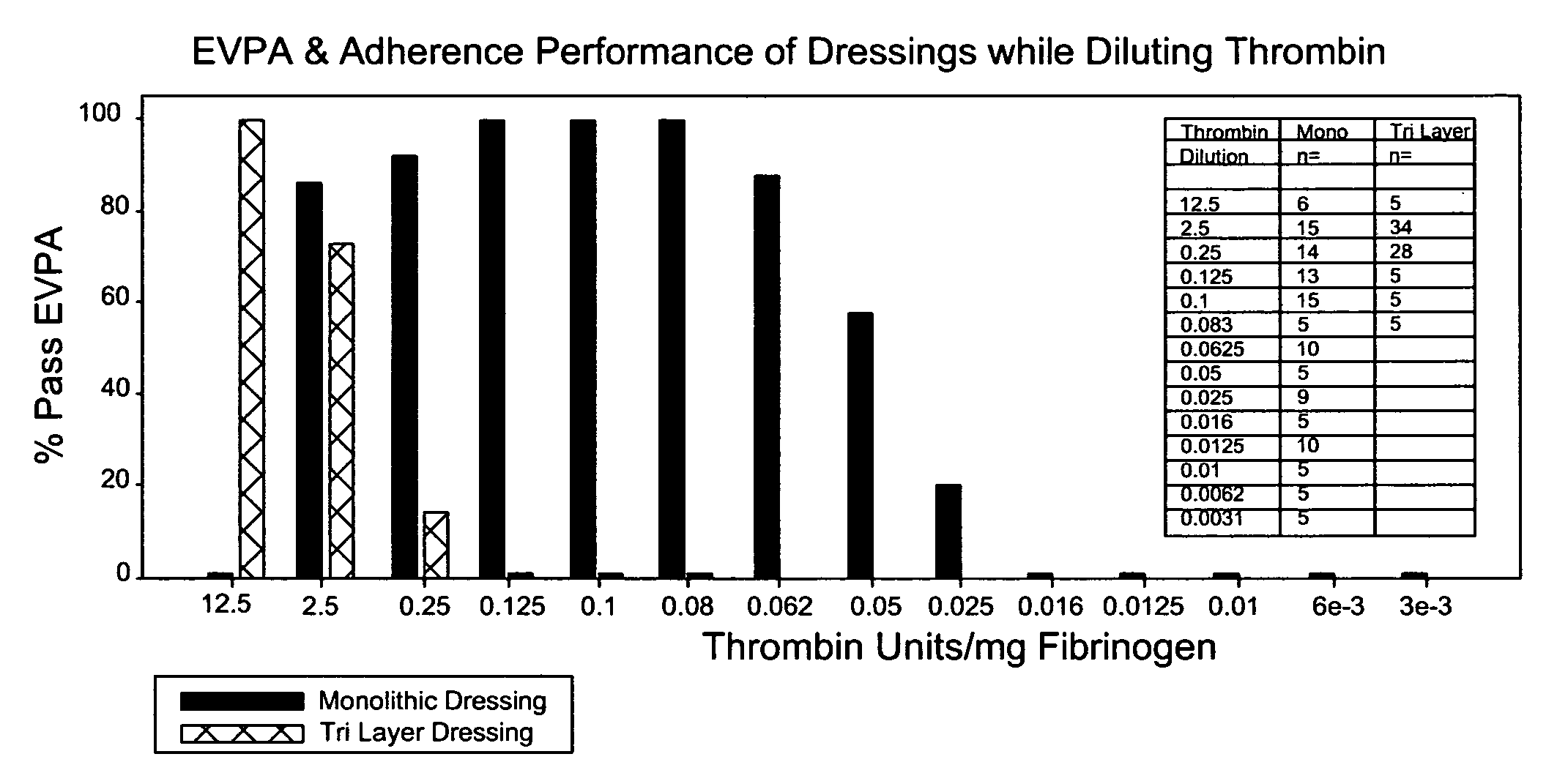
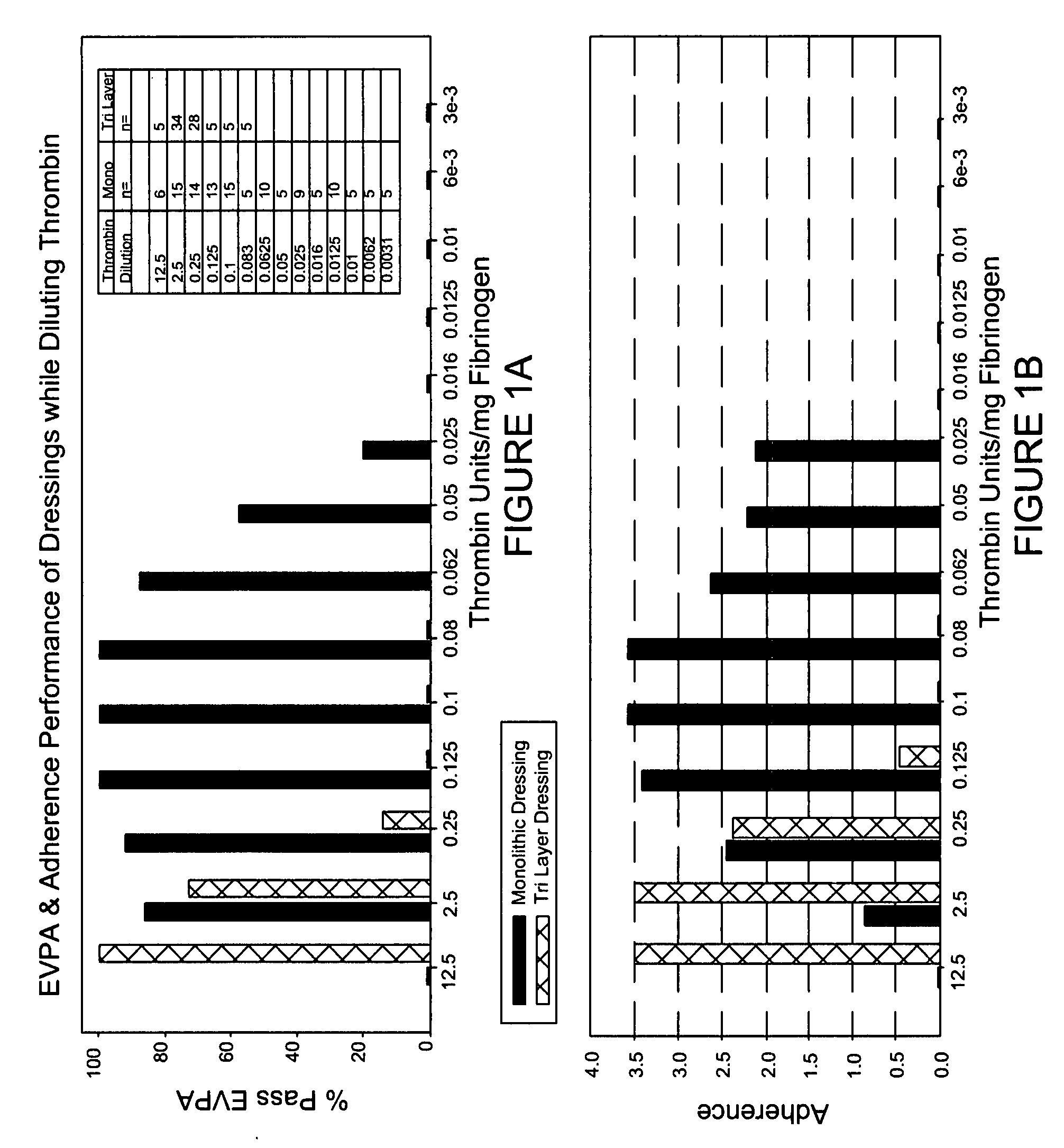
[0107] Backing material was placed into each 1.5×1.5 cm PVC molds. Fifteen microliters of 2% sucrose was pipetted on top of each of the four corners of the backing material. A second piece of PETG plastic was fitted on top of the 1.5×1.5 molds and held in place. This formed a closed mold. The molds were then placed in a −80° C. freezer for at least 60 minutes. Fibrinogen (ERL lot 3100) was formulated in CFB. The fibrinogen concentration was adjusted to 37.5 mg / ml using CFB. The final pH of the fibrinogen was 7.4±0.1. Once prepared the fibrinogen was placed on ice until use. Thrombin was formulated in CTB. The final pH of the thrombin was 7.4±0.1. The thrombin concentrations were adjusted using CTB to deliver the following amounts 2.5, 0.25, 0.1, 0.05, 0.025, 0.016, 0.0125 and 0.01 units / mg of Fibrinogen (upon mixing), which corresponded to 624, 62.4, 25, 12.5, 6.24, 3.99, 3.12, and 2.5 Units / ml thrombin prior to mixing. Once prepared the thrombin was placed on ice until use. The tem...
example 3
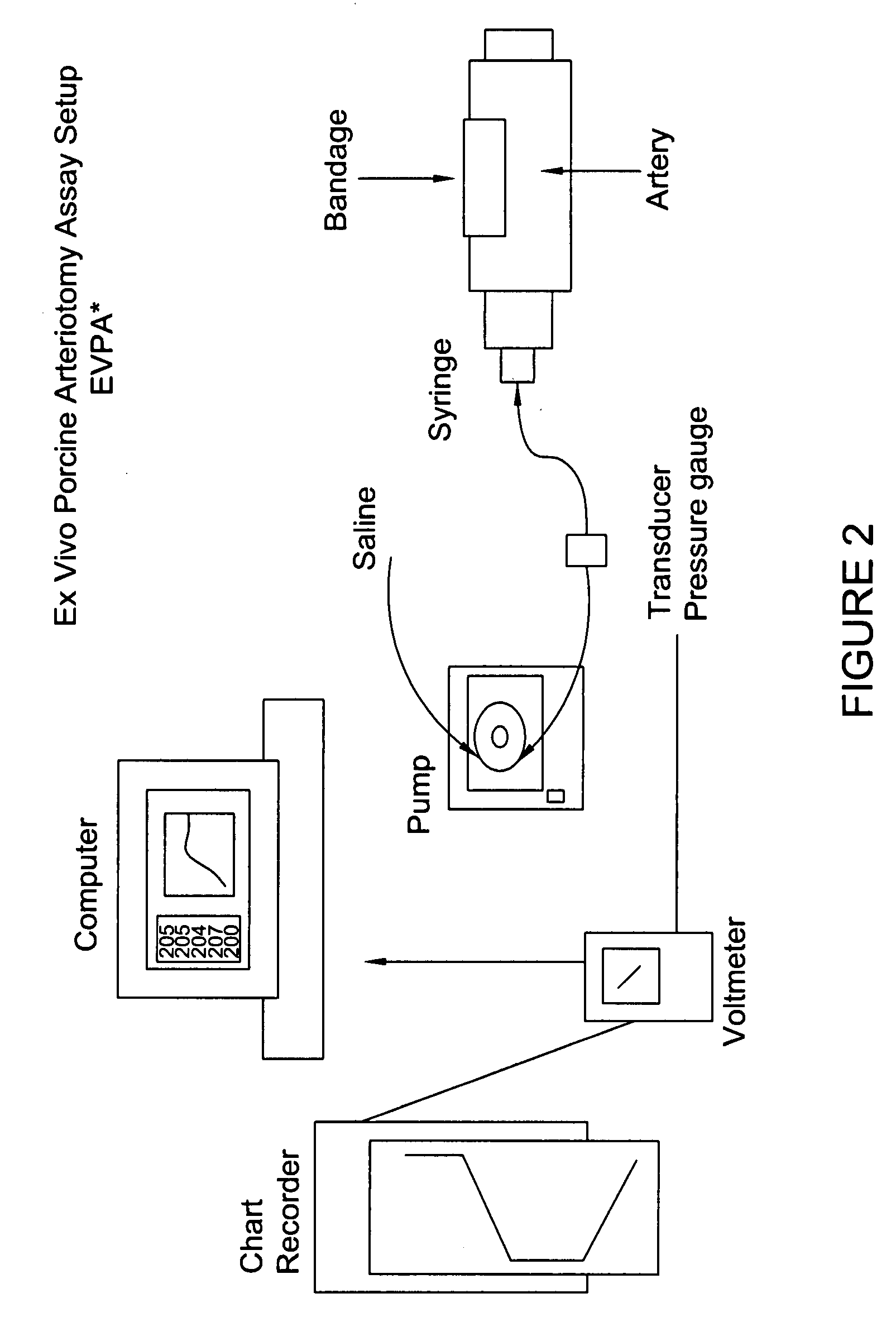
[0108] Backing material was placed into 2.4×2.4 cm PVC molds. Twenty-five microliters of 2% sucrose was pipetted on top of each of the four corners of the backing material. Once completed the molds were placed in a −80° C. freezer for at least 60 minutes. Fibrinogen (ERL lot 3100) was formulated in CFB. The fibrinogen concentration was adjusted to 37.5 mg / ml using CFB. The final pH of the fibrinogen was 7.4±0.1. Once prepared the fibrinogen was placed on ice until use. Thrombin was formulated in CTB. The final pH of the thrombin was 7.4±0.1. Using CTB, the thrombin concentrations were adjusted to deliver the following amounts 0.125, 0.025, 0.0125, 0.00625 and 0.0031 units / mg of Fibrinogen upon mixing, which corresponded to 31.2, 6.24, 3.12, 1.56 and 0.78 Units / ml thrombin prior to mixing. Once prepared the thrombin was placed on ice until use. The temperature of the fibrinogen and thrombin prior to dispensing was 4° C.±2° C. The molds were removed from the −80° C. freezer and placed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com