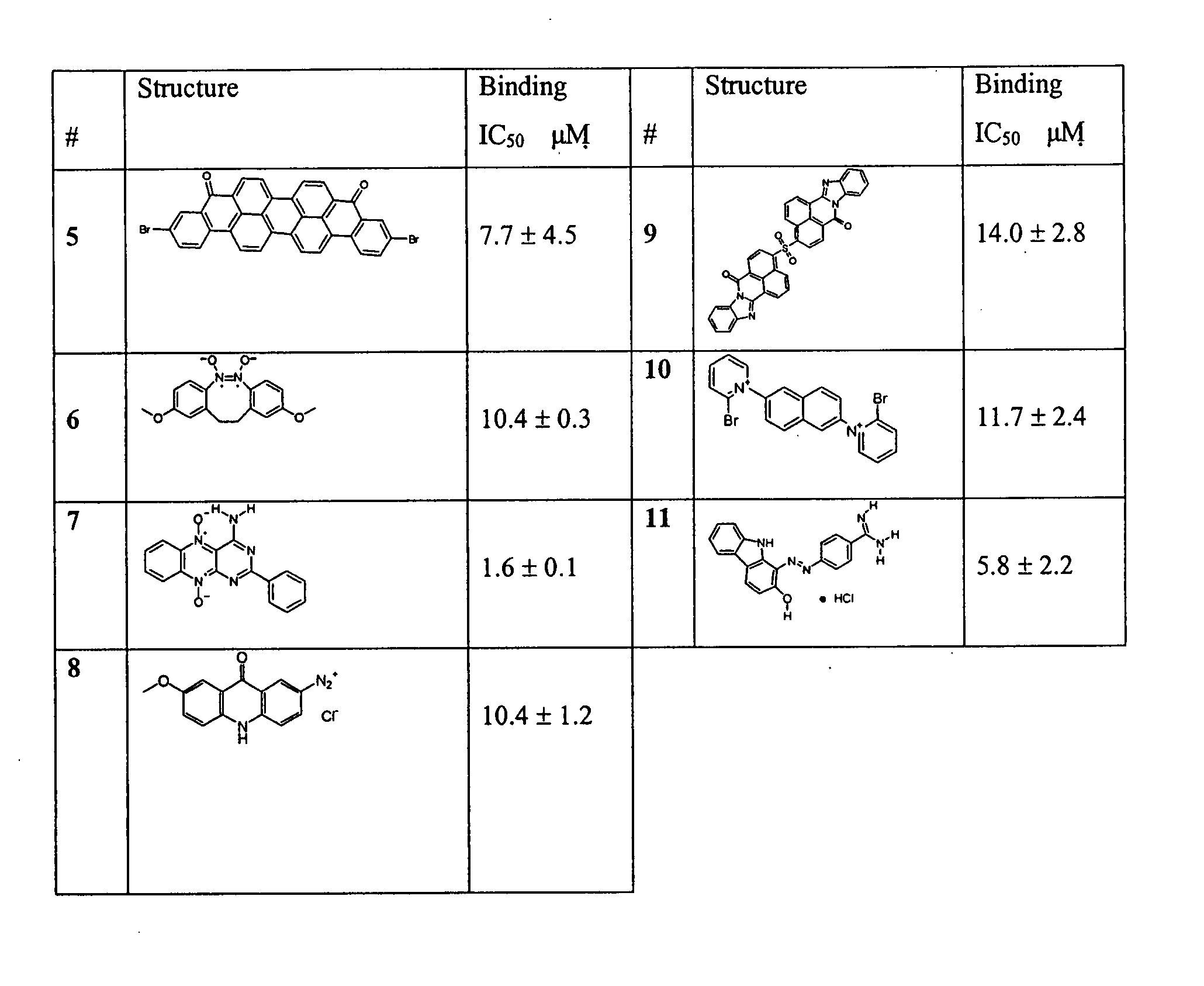
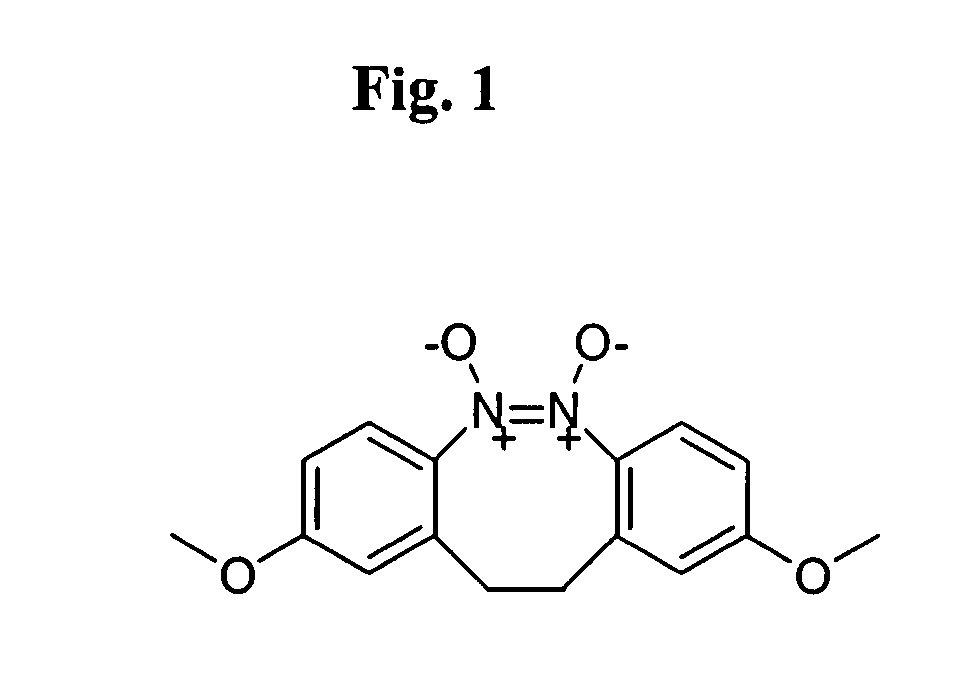
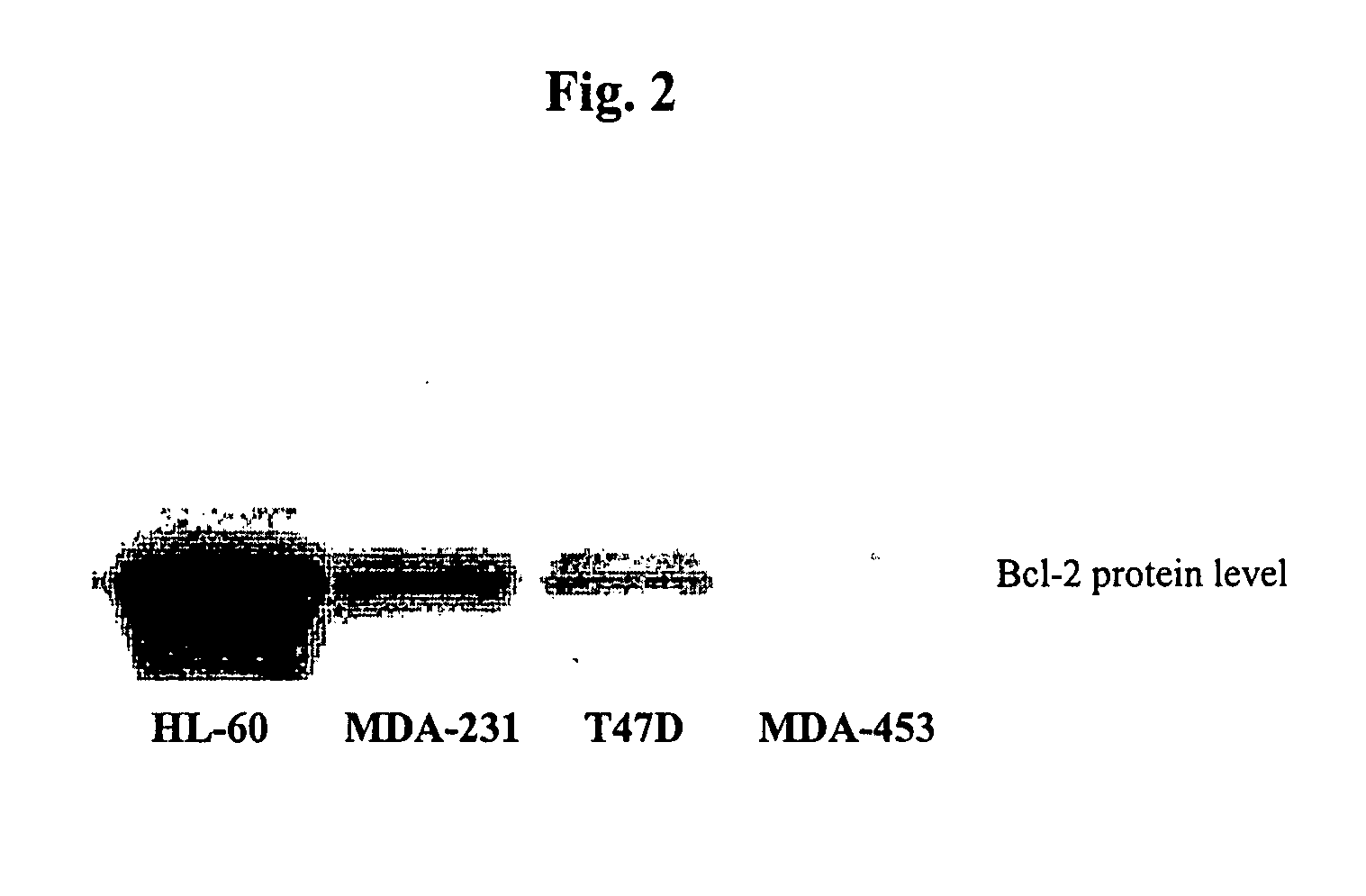
Small molecule inhibitors targeted at Bcl-2
a technology of bcl-2 and small molecule inhibitors, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of serious deleterious effects and diseases to arise in an organism, faulty regulation of the apoptotic machinery, and limited success in attempts to reduce the overexpression of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Homology Modeling
[0185] The sequence of human Bcl-2 was obtained from Gene Bank (entry gi4557355). The NMR structure of Bcl-XL (pdb code: 1BXL from the protein databank[40]), which has 45% amino acid sequence identity, 56% sequence similarity and 3% gaps with Bcl-2, was used as the template (Michael et al., Science, 275:983-986 [1997]). The structure of Bcl-2 was built using the homology-modeling program MODELLER (version 4.0). (See, MODELLER web site http: / / guitar.rockefeller.edu / modeller / modeller.html). MODELLER is most frequently used for comparative modeling of protein three-dimensional structure. More generally, MODELLER models protein 3D structure by satisfaction of spatial restraints (Sali et al., PROTEINS: Structure, Function, and Genetics, 23:318-326 [1995]; Sali, A. Curr. Opin. Biotech., 6:437451 [1995]). The restraints used in the comparative modeling of Bcl-2 structure were automatically derived from the experimental 3D structure of Bcl-XL by the MODELLER program. The o...
example 2
Structure-Based 3D-Database Searching
[0187] The refined structure of Bcl-2 from the Bak / Bcl-2 complex, obtained after the 3 ns MD simulation, was used for structure-based database searching of the NCI-3D database (Milne et al., J. Chem. Inf. Comput. Sci., 34:1219-1224 [1994]). Program DOCK (version 4.0.1) was employed (Ewing and Kuntz, J. Comput. Chem., 18:1175-1.189 [1997]). All residues within 8 Å from the Bak peptide were included in the binding-site used for screening. United atom KOLLMAN charges were assigned for the protein using the BIOPOLYMER menu in the Sybyl program (Sybyl, Tripos, Inc., St. Louis, Mo.). Because of its general applicability, the Geisterger method as implemented in Sybyl was used to assign charges to the compounds. National Cancer Institute's 3D database of 206,876 small molecules and natural products that can be accessed by the public (Milne et al., J. Chem Inf. Comput. Sci., 34:1219-1224 [1994]) was searched.
[0188] The interactions between the Bak BH3 p...
example 3
Chemical Samples of the “Open” Compounds
[0189] All 35 chemical samples were dissolved at 10 mM in dimethyl sulfoxide (DMSO) prior to biological experiments.
[0190] A. Chemistry:
[0191] All chemical reagents were commercially available. Melting points were determined on a MelTemp II apparatus and are uncorrected. Silica gel chromatography was performed on silica gel 60, 230-400 mesh (E. Merck). 1H spectra were recorded on a Varian Mercury instrument at 300 MHz or on a Bruker AC-250 instrument at 250, and 13C NMR Spectra were recorded on a Bruker AC-250 instrument at 62.9 MHz. Spectra were referenced to the solvent in which they were run (7.24 ppm for 1H CDCl3). Elemental analyses were performed by Atlantic Microlab, Inc., Atlanta, Ga. FAB mass spectra were performed on a VG-7070-EHF Mass Spectrometer, at unit resolution (isotopic mass), in the positive and / or negative ion mode. Sample matrix: 3-nitrobenzyl alcohol (NBA).
[0192] B. Analytical Analysis of the Compounds:
[0193] Compoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com