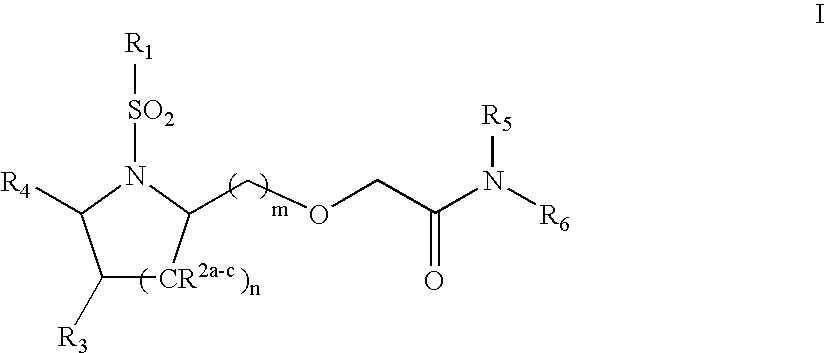
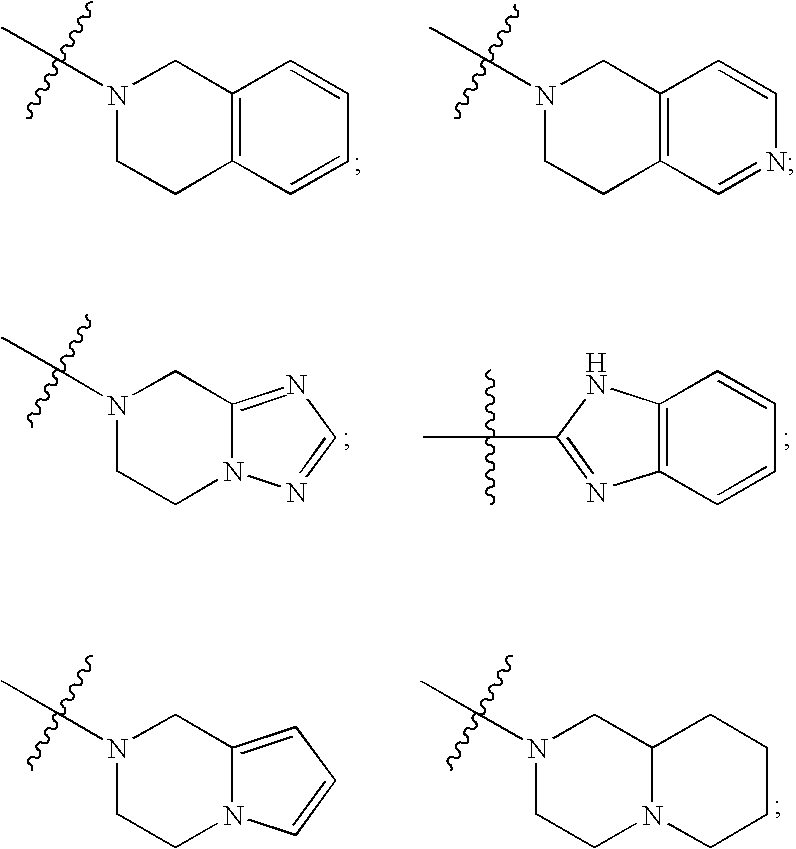
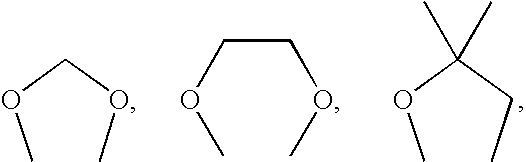
Substituted sulfonamide compounds
a technology of sulfonamide and derivatives, which is applied in the direction of drug compositions, antibacterial agents, metabolic disorders, etc., can solve the problems of increasing the cost of transgenic animals, increasing the cost of working with transgenic animals, and increasing the cost of working with unchanged animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 8
2-[1-(4-methoxy-2,6-dimethyl-phenylsulfonyl)-piperidin-2-ylmethoxy]-1-[4-(1-methyl-piperidin-4-yl)-piperazin-1-yl]-ethanone
[0761]
[0762]N,N′-Carbonyldiimidazole (114 mg, 0.706 mmol) was added to a solution of 2-((1-(4-methoxy-2,6-dimethylphenylsulfonyl)piperidin-2-yl)methoxy)acetic acid (250 mg, 0.673 mmol) in dichloromethane (15 ml), and the mixture was stirred for 1 h at room temperature. A solution of 1-(1-methylpiperidin-4-yl)piperazine (123 mg, 0.673 mmol) in dichloromethane (5 ml) was then added, and the reaction mixture was stirred for 15 h at room temperature. The reaction mixture was then extracted with water (20 ml) and saturated sodium chloride solution (20 ml), and the organic phase was dried over magnesium sulfate and concentrated in vacuo. The crude product was purified by flash chromatography using dichloromethane / methanol (97:3→90:10).
[0763]Yield: 296 mg (82%), brown resin
[0764]1H-NMR (600 MHz, DMSO-d6): 1.27 (1H); 1.42 (1H); 1.55 (4H); 1.69 (2H); 1.79 (1H); 1.89 (2H)...
example 97
2-((1-(4-methoxy-2,6-dimethylphenylsulfonyl)piperidin-2-yl)methoxy)-1-(4-(1-methyl-piperidin-4-yl)piperazin-1-yl)ethanone dihydrochloride
[0765]N,N′-Carbonyldiimidazole (272 mg, 1.696 mmol) was added to a solution of 2-((1-(4-methoxy-2,6-dimethylphenylsulfonyl)piperidin-2-yl)methoxy)acetic acid (600 mg, 1.615 mmol) in dichloromethane (15 ml), and the mixture was stirred for 1 h at room temperature. A solution of 1-(1-methylpiperidin-4-yl)piperazine (293 mg, 1.615 mmol) in dichloromethane (5 ml) was then added, and the reaction mixture was stirred for 15 h at room temperature. Saturated sodium hydrogen carbonate solution (20 ml) was then added to the reaction mixture, and then the aqueous phase was extracted with dichloromethane (2×20 ml). The combined organic phases were extracted with saturated sodium chloride solution (20 ml), dried over sodium sulfate and concentrated in vacuo. The crude product was purified by flash chromatography using dichloromethane / methanol (5:1). 2-((1-(4-me...
example 92
3-((4-(2-((1-(4-methoxy-2,6-dimethylphenylsulfonyl)piperidin-2-yl)-methoxy)acetyl)piperazin-1-yl)methyl)benzonitrile hydrochloride
[0768]
[0769]N,N′-Carbonyldiimidazole (68 mg, 0.424 mmol) was added to a solution of 2-((1-(4-methoxy-2,6-dimethylphenylsulfonyl)piperidin-2-yl)methoxy)acetic acid (150 mg, 0.404 mmol) in dichloromethane (4 ml), and the mixture was stirred for 1 h at room temperature. A solution of 3-(piperazin-1-ylmethyl)benzonitrile (81 mg, 0.404 mmol) in dichloromethane (1 ml) was then added, and the reaction mixture was stirred for 15 h at room temperature. Saturated sodium hydrogen carbonate solution (5 ml) was then added to the reaction mixture, and then the aqueous phase was extracted with dichloromethane (2×10 ml). The combined organic phases were extracted with saturated sodium chloride solution (10 ml), dried over sodium sulfate and concentrated in vacuo. The crude product was purified by flash chromatography using ethyl acetate / hexane (20:1). 3-((4-(2-((1-(4-met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com