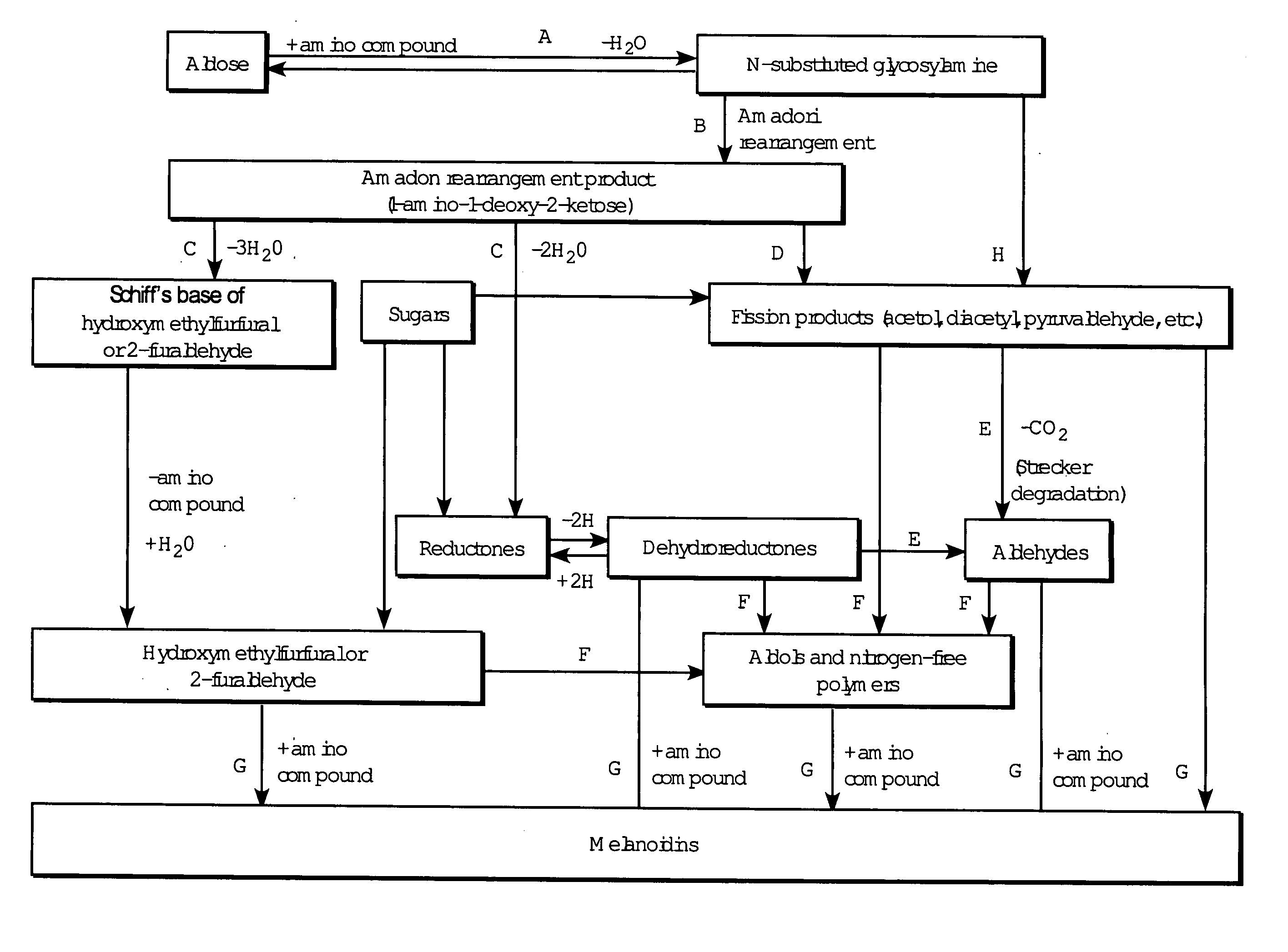
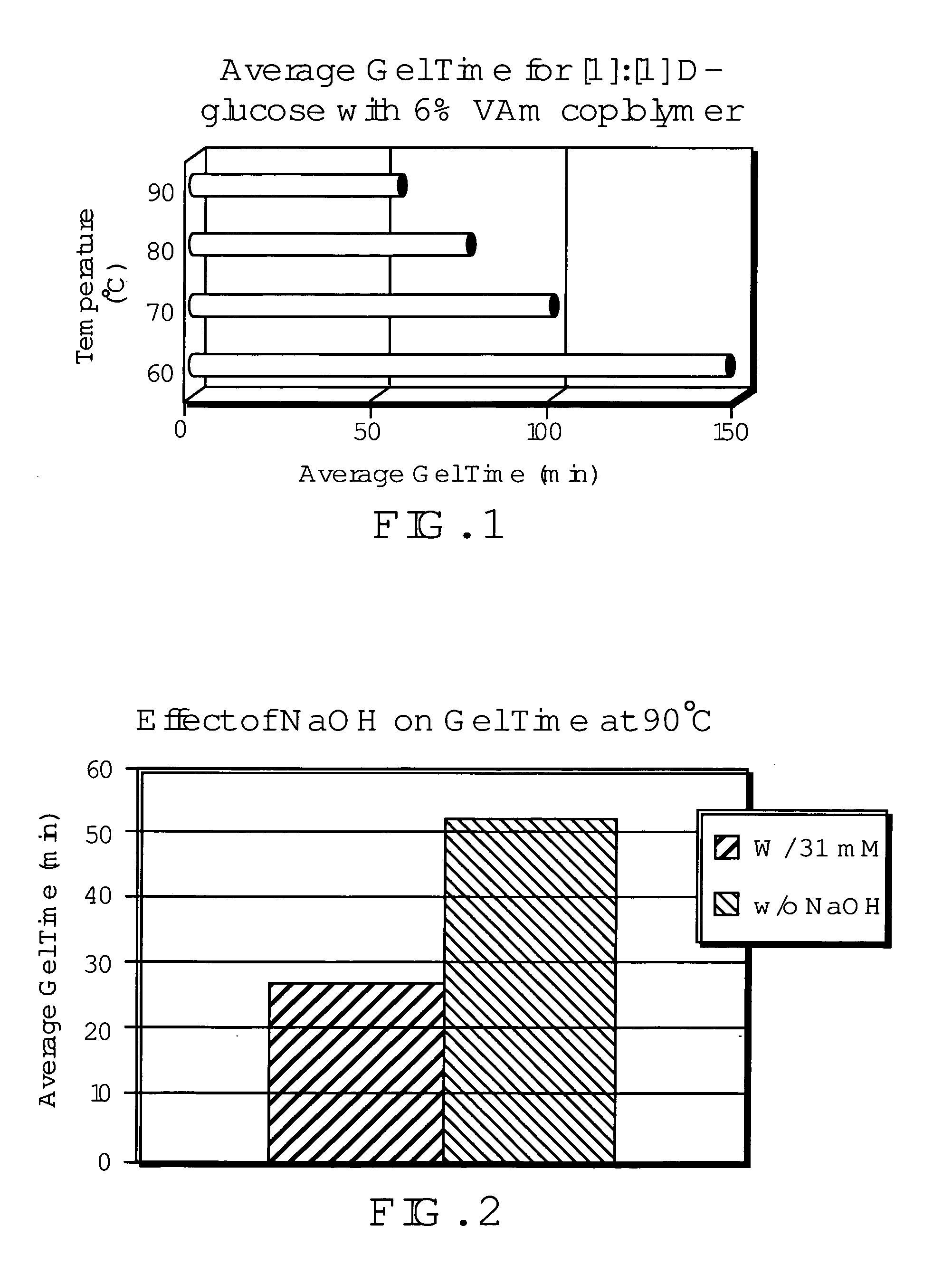
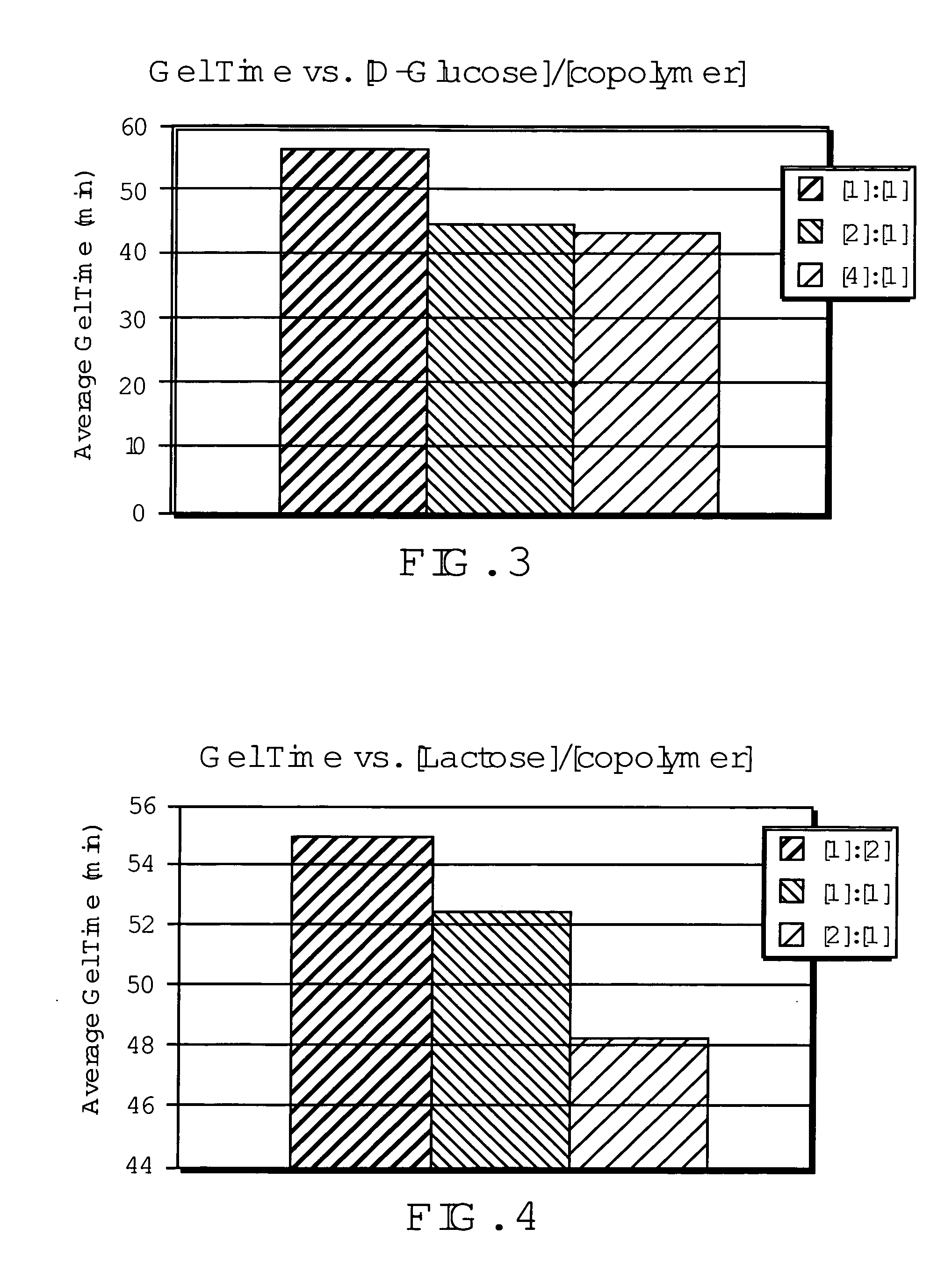
Networked polymeric gels and use of such polymeric gels in hydrocarbon recovery
a polymeric gel and network technology, applied in fluid removal, chemistry apparatus and processes, borehole/well accessories, etc., can solve the problems of difficult implementation of the above definition in practice, and it is almost impossible to measure the total dissolving material in practice, so as to increase the amine content and stiffen the resultant gel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047]An aqueous solution was prepared by dissolving 7.5 g D-glucose and 2.5 g poly(vinylalcohol) (PVOH) into distilled, deionized water in a 25 mL volumetric flask. The solution was clear with some undissolved polymer. It was, however, pourable. The solution was transferred to a round bottom flask and heated to 80° C. in an oil bath. Heating was done with constant stirring and under reflux conditions. Upon completion the solution remained clear with all polymer dissolved and was still pourable.
example 2
[0048]Prior studies suggest that an aqueous solution of PVOH and D-glucose could be used to form hydrogels by using freezing / thawing cycles. See Yamaura, K.; Fukada, M.; Tanaka, T.; Tanigami, T. J. of Applied Polymer Science. 1999, 74, 1298-1303. To study this effect, a solution was prepared as in example 1. Heating was carried out using the same procedure as in example 1, but was allowed to reach a temperature of 90° C. The aqueous solution was then placed in a −10° C. freezer over 48 hours. After thawing the solution at room temperature for 1 hour a weak, white hydrogel had formed. The gel was then placed back in the freezer for 24 hours and then thawed at room temperature for 1 hour. After which, the gel appeared visibly stronger. This gel was found to be soluble in water heated up to 49° C. Neither swelling nor dissolution was noted when placed in 1M HCl.
example 3
[0049]Prior studies further suggest that D-glucose was not necessary for the gelation of poly(vinylalcohol) using the process in example 2. See Yamaura, K.; Karasawa, K. I.; Tanigami, T.; Matsuzawa, S. J. of Applied Polymer Science. 1994, 51, 2041-2046. To study such gelation, a 2.5 g of PVOH was dissolved in distilled, deionized water in a 25 mL volumetric flask. Heating was carried out using the same procedure as in example 1, but was allowed to reach a temperature of 95° C. The solution was then placed in the freezer at −25° C. for 48 hours. After 1 hour of thawing at room temperature a gel, similar in appearance to the gel in Example 2, was produced. The inability of PVOH to form hydrogels without the freezing / thawing cycle indicated that the amine groups on copolymers of PVOH and Poly(vinylamine) in the compositions of the present invention are responsible for gelation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com