Systems and methods for multiplex analysis of PCR in real time
a real-time, multiplexing technology, applied in the field of systems and methods, can solve the problems of limited difficulty in quantifying the starting template, and unparallel amplification and precision capability, and achieve the effect of increasing the multiplexing capability of real-time pcr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1. Example 1
The Stability of Magnetic Microspheres Under PCR Cycling Conditions
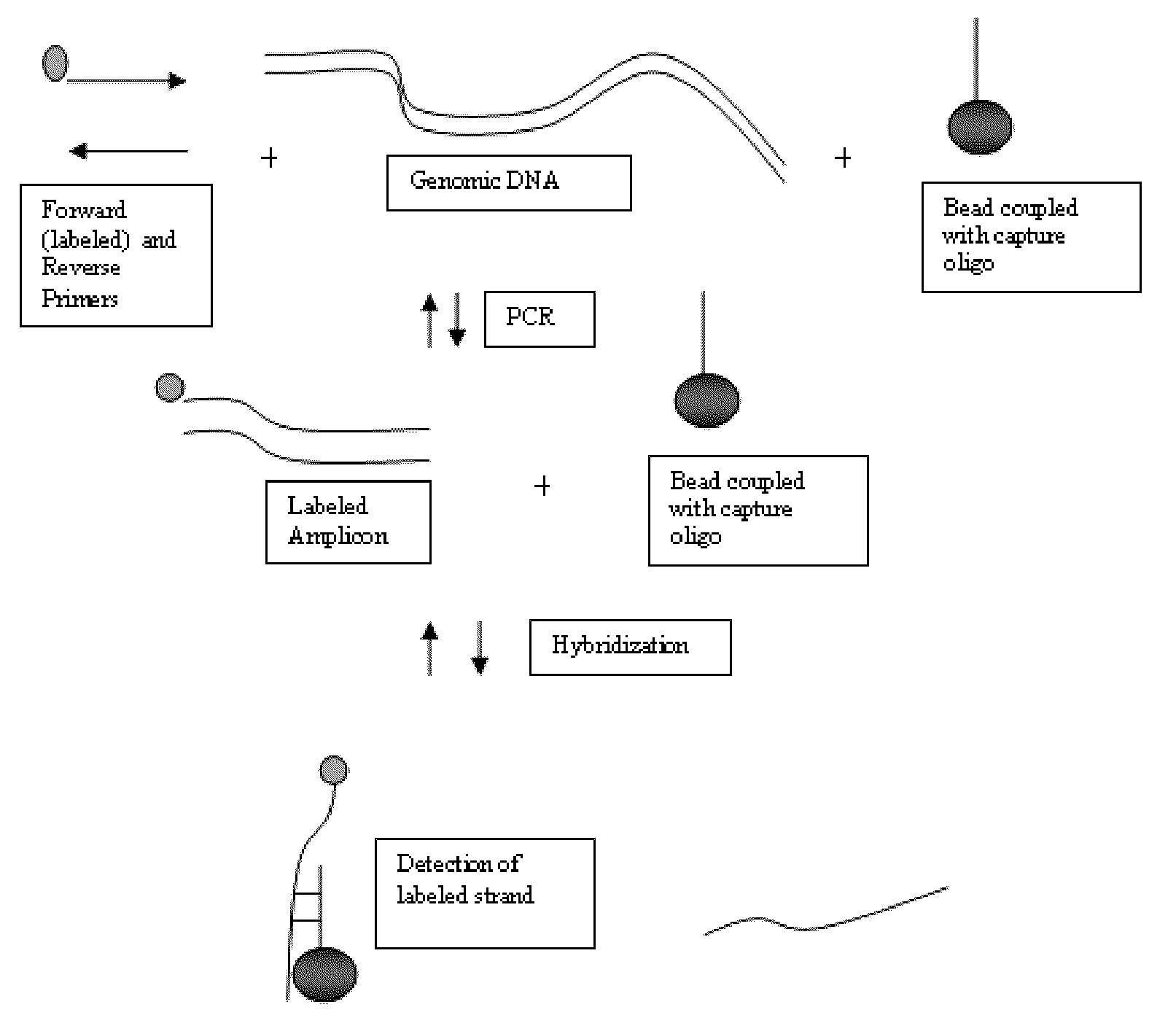
[0184]This study demonstrates the robust stability of the Luminex super paramagnetic microspheres in PCR cycling conditions. It also demonstrates that an amplicon can be captured and detected at various stages of the PCR reaction as the concentration thereof increases. This study also shows that a two-probe system is a viable design for real-time PCR detection using magnetic microspheres. Hybridization probes (US 2001 / 6174670) also use a two-probe system.
[0185]Experimental Design: A two probe system was used to detect the generation of a Factor V gene amplicon while in the presence of magnetic microspheres that were coupled with target specific nucleotide probes. A target specific probe (FV Probe Ano) was attached to bead set 22. This probe was not fluorescently labeled. Another probe was included in the mix, which was not attached to a bead set, but was target specific, and labeled with a Cy3 fluorophore...
example 2
2. Example 2
Amplification Detection Using Tagged Primers
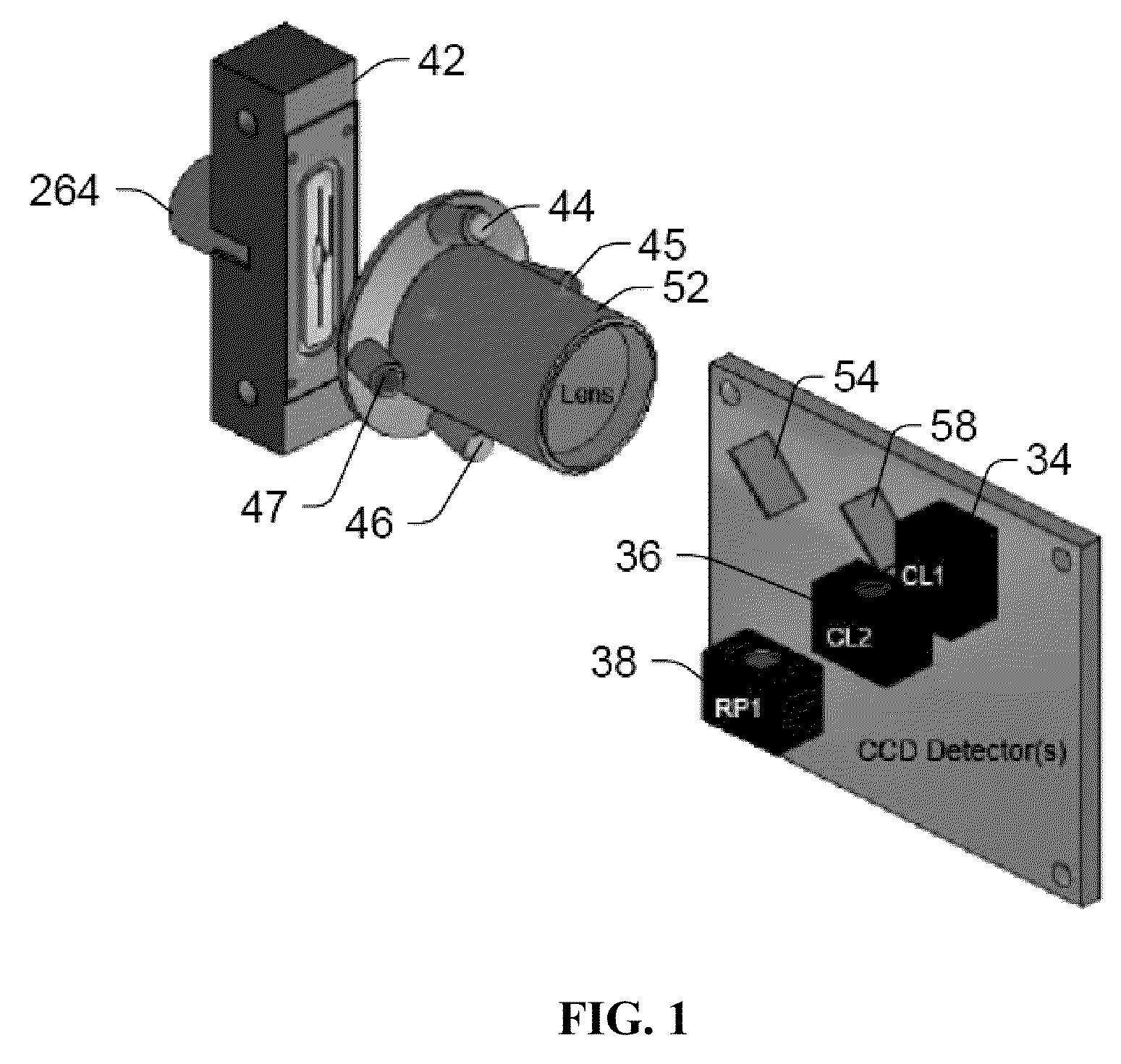
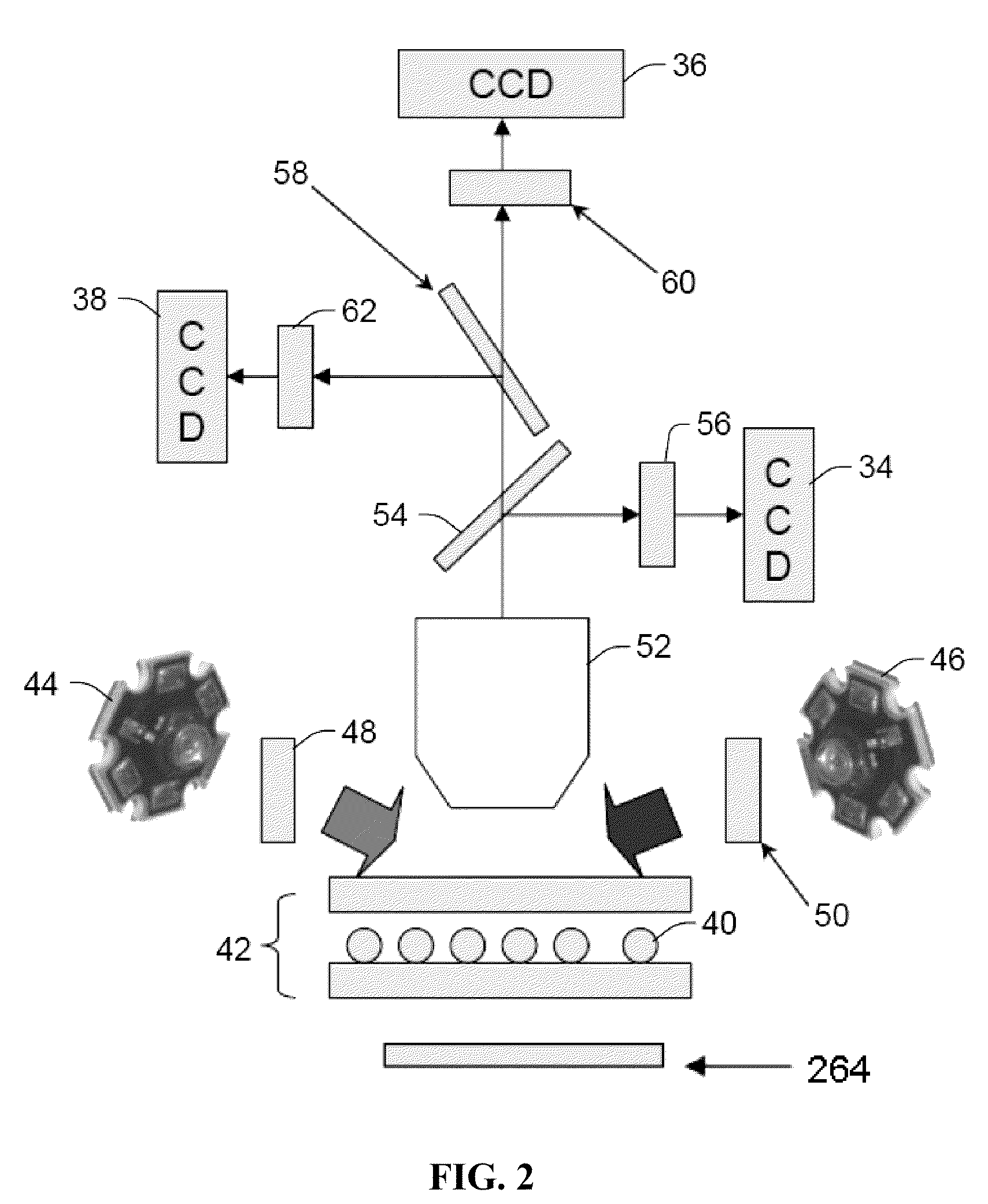
[0223]The following example demonstrates that a nucleic acid amplification signal can be measured in real-time using the tagged primer method. This example also demonstrates that these measurements can be performed using a imaging system comprising a quartz imaging chamber and a magnet that can be moved adjacent to the quartz imaging chamber to magnetically pull the superparamagnetic particles to a two dimensional surface of the chamber opposite a charge coupled device (CCD) detector (see e.g., FIGS. 1 and 2) in the presence of, and without modification to, the Polymerase Chain Reaction solution, and that the measurements thereof are comparable to the Luminex 200 system. The imaging system comprising the quartz chamber and magnet may be considered a “static” imaging system since the detector takes an image of the particles and any molecules bound to them while they are immobilized on the surface of the chamber by the magnetic f...
example 3
3. Example 3
Multiplex of 8
[0238]In this example, the Direct Hybridization method was used with 8 primer sets and 14 bead sets. An excess of bead sets were used to show that the amplification products do not non-specifically hybridize to bead sets containing unrelated probes.
[0239]A PCR cocktail was made including the following reagents:
TABLE 41x vol. μL45Qiagen hotstart plus 2xMaster251125MixH2O Ambion nuclease freeH2O177658 plex ordered from IDTPrimer145Coriell Sample 13033Template145Luminex MagPlexBeads3135microspheres50 mM mgCl2 Qiagen3135total502250
[0240]This cocktail, including the Luminex MagPlex microspheres with probe sequences attached thereto, was aliquoted into 40 PCR tubes at 50 μL each. Then 20 of the reactions had approximately 100 ng of genomic DNA added to them. These reactions were cycled on a BioRad iCycler thermal cycler. The primers were designed to amplify specific regions of the cystic fibrosis CFTR gene. Primer sequences for each of 8 primer sets used for this...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com