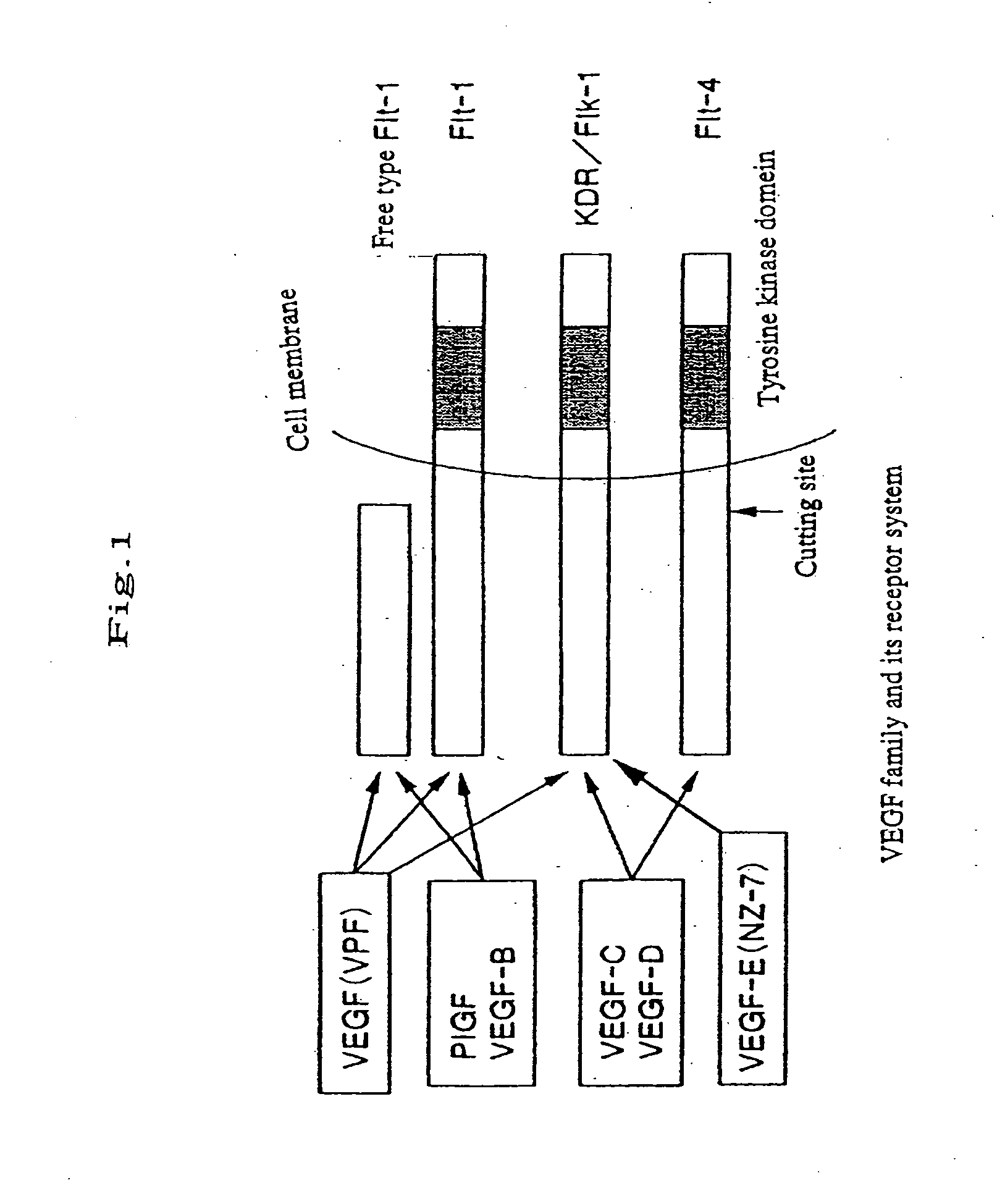
Chimera humanized vascular endothelial growth factor
a growth factor and humanized technology, applied in the field of chimera vascular endothelial growth factor, can solve the problems of disturbing vascularization therapy, causing patients to die, and causing a great deal of pain to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cells and Culture Conditions
[0113]Ex-Cell 400 (JRH Biosciences, Lenexa, Kans.) was used as a culture solution for Sf9 insect cells (Invitrogen, CA, USA). NIH3T3 mouse fibroblast and a cell strain NIH3T3-KDR which strongly expresses a human VEGF receptor, KDR (VEGFR-2), were used in the experiment for assaying the KDR autophosphorylation by ligand binding. The NIH3T3-KDR cell was produced by Sawano et al. (Sawano A. et al. Cell growth and Differentiation, 7, 213-221, 1996). The NIH3T3 cell and the NIH3T3-KDR cell were maintained in a medium obtained by adding 10% bovine serum, 2 mM L-glutamine, and 200 μg / ml G418 (Geneticin; Life Technologies, Inc., Grand Island, N.Y.) to Dulbecco's Modified Eagle's Medium (DMEM, Nissui, Tokyo). Human umbilical vein endothelial cells (HUVEC, Morinaga, Tokyo) were maintained in HUVE culture medium (Morinaga, Tokyo) and used in the assay for the endothelial cell growth. Recombinant human VEGF165 was forcibly expressed in the Sf9 cells by baculovirus sy...
example 2
Production of Mutant Chimera VEGF-EN27 Proteins
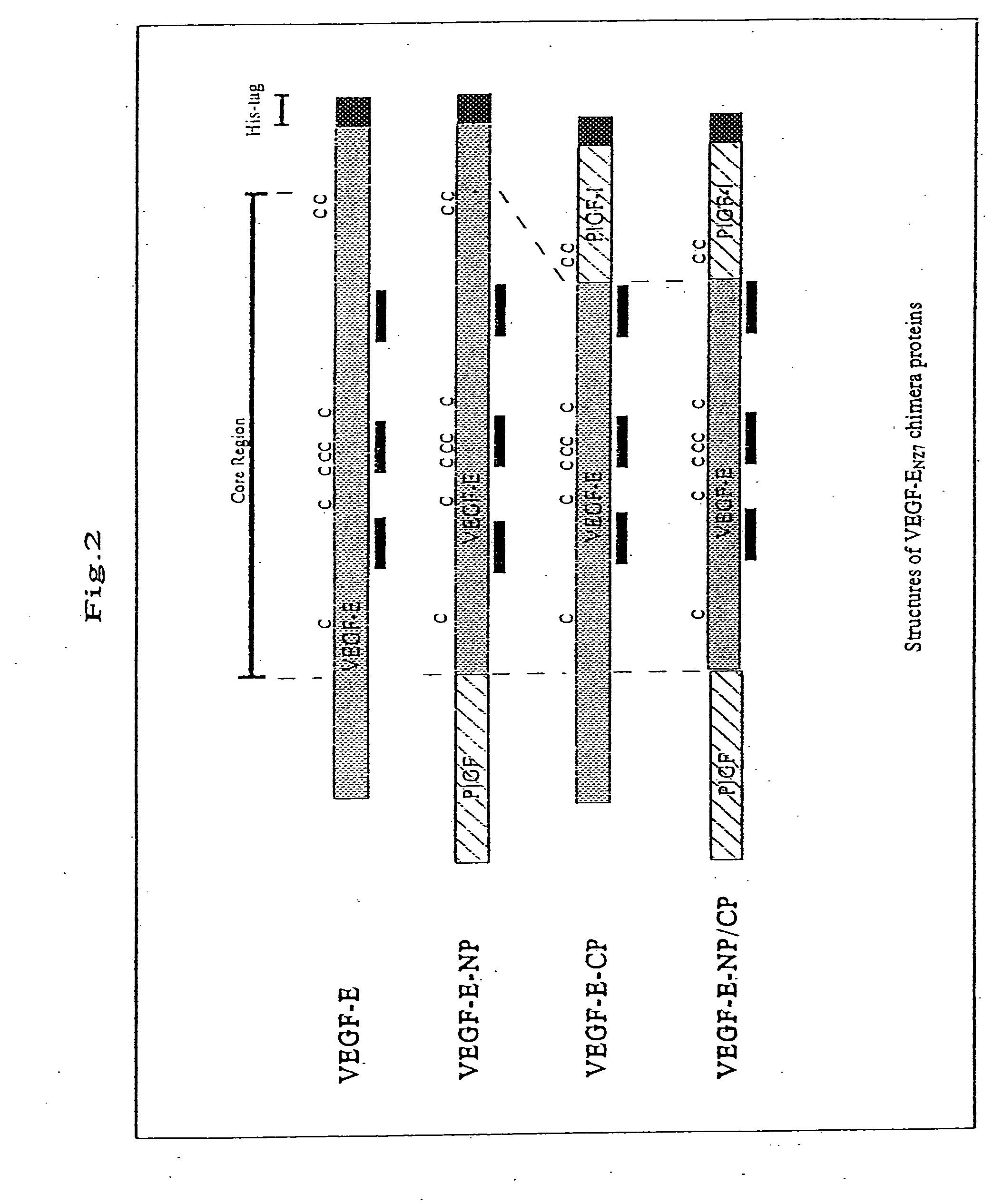
VEGF-E-NP, VEGF-E-CP, VEGF-E-NP / CP
[0114]The following three types of VEGF-EN27 / human PIGF-1 (hereinafter referred to as “hPIGF-1”) chimera proteins were produced (FIG. 2):
[0115](1) chimera protein VEGF-E-NP prepared by substituting 34 amino acid residues at the amino terminus of VEGF-EN27 with 40 amino acid residues at the amino terminus of hPIGF-1;
[0116](2) VEGF-E-CP prepared by substituting 18 amino acid residues at the carboxyl terminus of VEGF-EN27 with 21 amino acid residues at the carboxyl terminus of hPIGF-1; and
[0117](3) VEGF-E-NP / CP prepared by substituting both the amino and carboxyl terminuses of VEGF-EN27 with those of hPIGF-1.
[0118]The above three types of chimera proteins were fused with histidine tags to simplify the purification. The method in which DNA encoding each chimera protein was prepared and subcloned into vector pUC18 is described below.
[0119]The nucleotide sequence and the amino acid sequence of VEGF-E-His are ...
example 3
Preparation of VEGF-E Chimera Protein
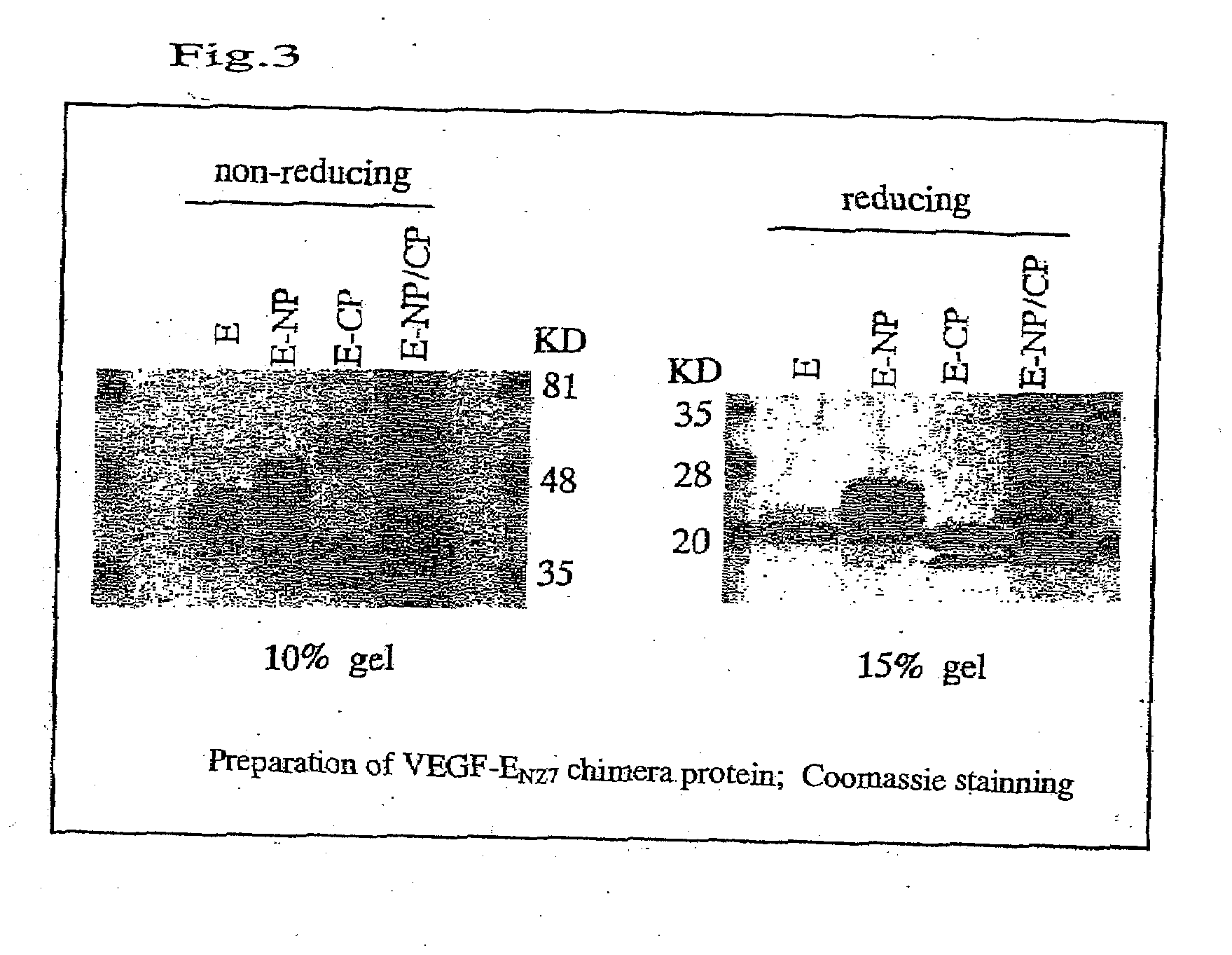
[0124]By using DNAs of pVL3E-CP, pVL3E-NP and pVL3E-NP / CP and Baculogold (Pharmingen, San Diego, Calif.) of baculovirus DNA, transfection into Sf9 cells was performed by lipofectin met Recombinant viruses having DNA encoding the chimera protein were amplified by repeating incubation for 3 to 4 days. In order to express the chimera protein, infection with the recombinant virus Sf9 cells was carried out at the multiplicity of infection (MOI) (i.e., the number of virus particles per cell) of 10. After the incubation for 3 days, a culture solution to which the chimera protein had been secreted was collected.
[0125]Subsequently, the culture solution was concentrated. In order to concentrate 150 ml of culture solution to 15 ml, an agitation-type ultrafilter device (MILLIPORE, USA) was used. The concentrate was subjected to dialysis in 20 mM sodium phosphate buffer (pH 8.0), 10 mM imidazole, 300 mM NaCl, and 20% glycerol in order to negatively charge the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| humidity | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com