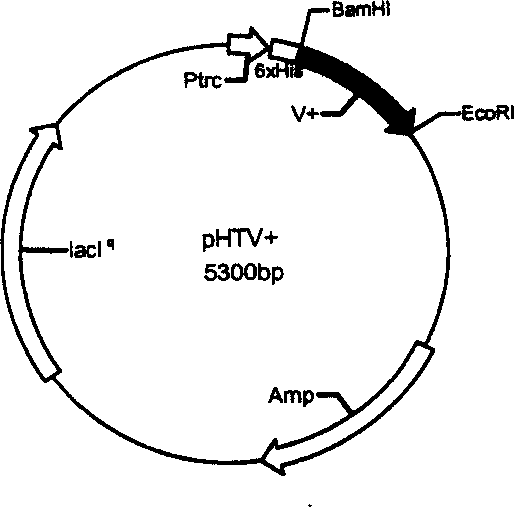
Recombinant protein molecule for inhibiting attack and transfer of cancer cell and angiogenesis
A technology of malignant tumor and angiogenesis, applied in peptide/protein components, anti-tumor drugs, recombinant DNA technology, etc., can solve problems affecting endothelial cell function, single function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] (1) PCR method to obtain V+DNA molecule
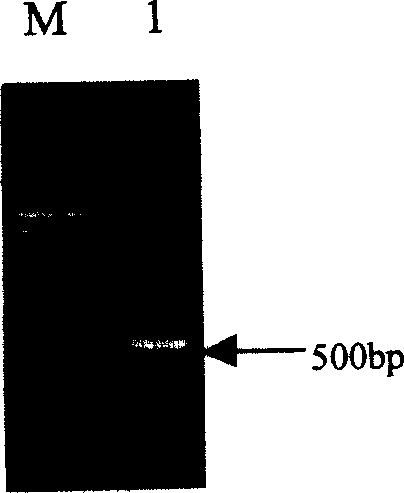
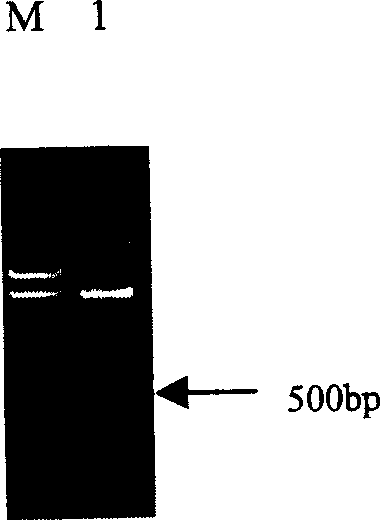
[0045] Design primers according to the 23rd N-terminal amino acid sequence of VEGI and the C-terminal amino acid sequence, and the upstream primer is: 5'-GCG GGATCC ATGTGTACAACTCACTGGGGTTTCACACTTTGCCAAATGGCTCCAGTTGTGAGACAAACT-3', containing 3 protective bases, BamHI site (underlined), start codon, 10 mutated amino acid CTTHWGLTLC corresponding codons, spacer sequence QMPP corresponding codons and amino acid coding sequence after position 23 of VEGI. The downstream primer is 5'-CGC GAATTC GATATTTGCTCTCCTCTATAG-3' is a sequence complementary to the C-terminal coding sequence of VEGI, and an EcoRI site (underlined) is introduced. Human genomic DNA was used as a template for PCR amplification. The PCR reaction conditions were: 98°C denaturation for 5 minutes, 94°C for 40 seconds, 60°C for 60 seconds, 72°C for 60 seconds, a total of 30 cycles, and 72°C for 10 minutes. After PCR amplification, a 500bp V+ band ( figure 1 ).
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com