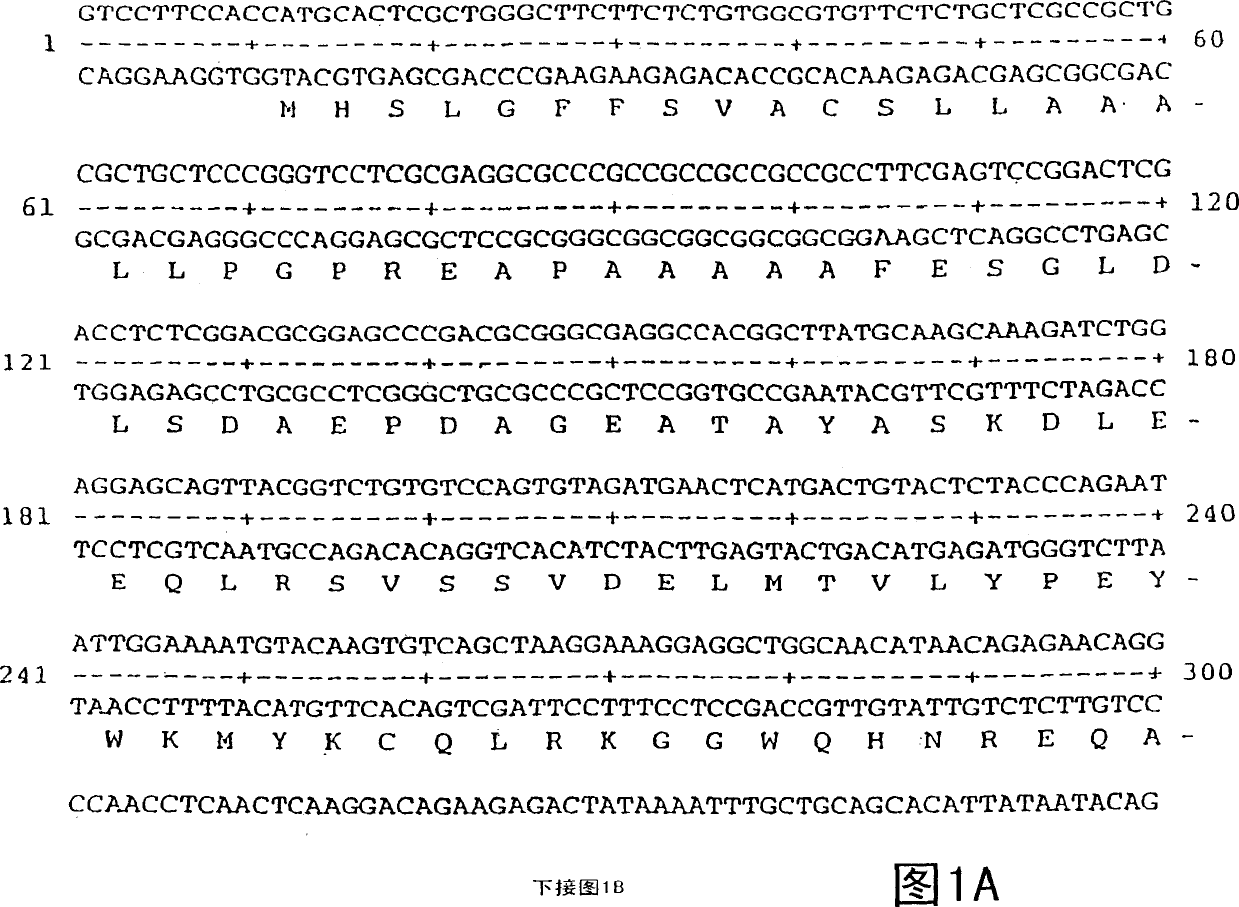
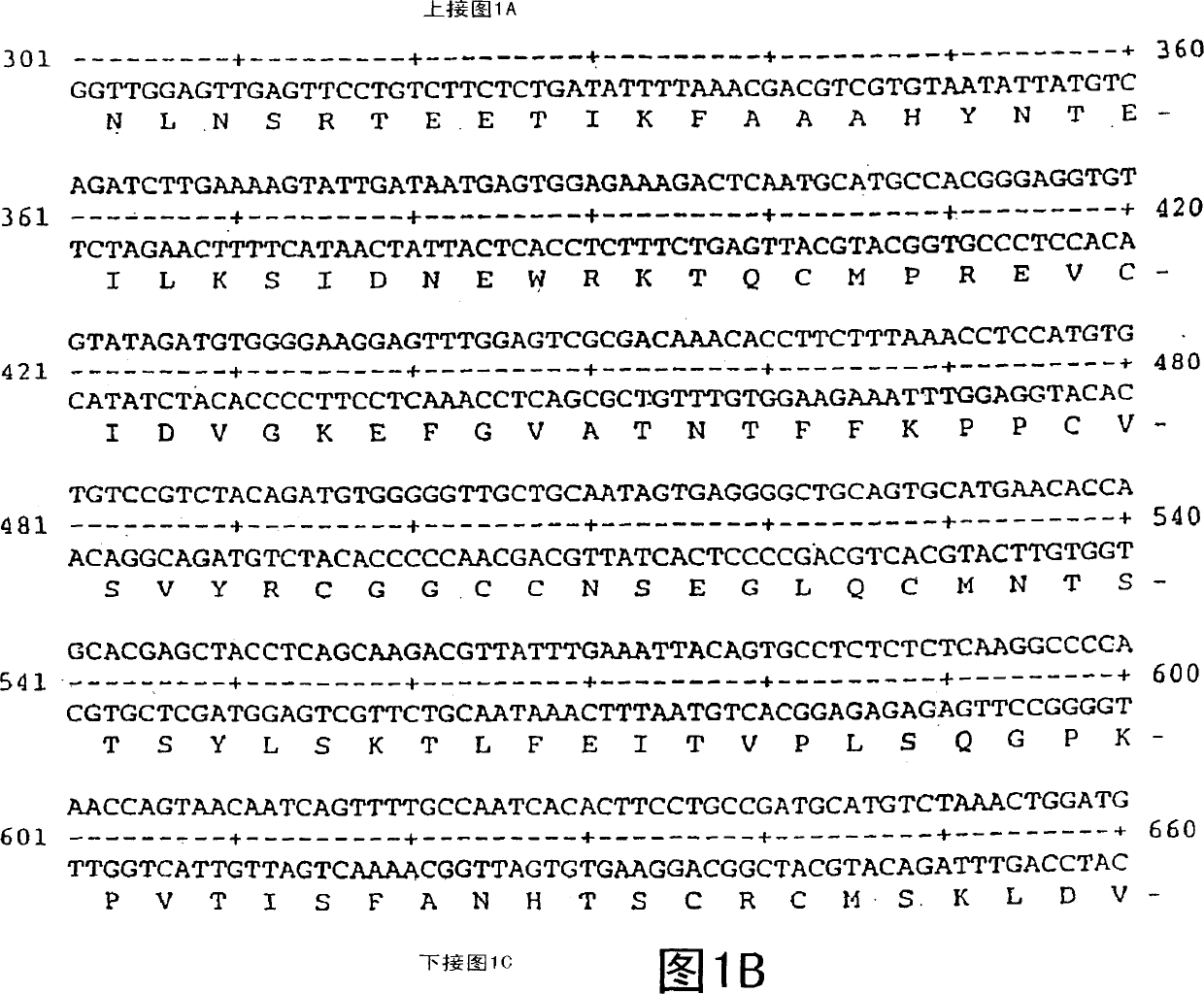
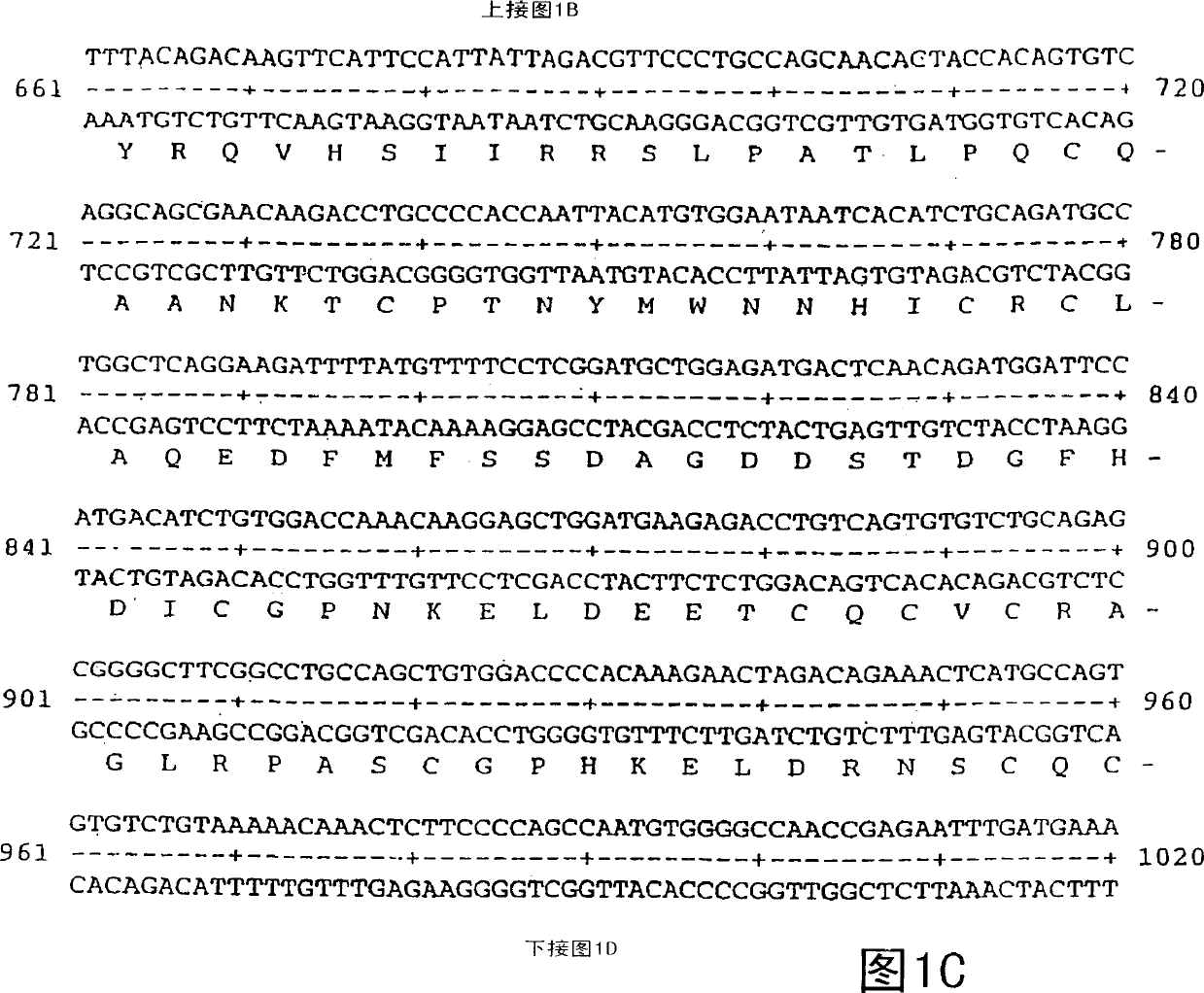
Vascular endothelial growth factor 2
A technology of nucleic acid molecules and myocardial ischemia, applied in the fields of epidermal growth factor, growth factor/inducer, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0413] Expression patterns of VEGF-2 in human tissues and breast cancer cell lines
[0414] Northern blot analysis was used to detect the expression level of VEGF-2 in human tissues and human breast cancer cells. RNAzol TMTotal RNA samples from cells were isolated by System B (Biotecx Laboratories, Ltd.). Use 1% agarose gel to separate the total amount of about 10 mg of RNA isolated from each breast cancer tissue and specific cell line, and spot it on a nylon filter, [Sambrook et al., Molecular Cloning: A Laboratory Manual, p. Second Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York (1989)]. Labeling reactions were performed with 50 ng of DNA fragments according to the Stratagene Prime-It kit. Labeled DNA was purified from 5Prime-3Prime using Select-G-50 columns, Ltd. [Boulder, CO]. The filter was hybridized with the full-length VEGF-2 gene labeled with a radioactivity of 1,000,000 cpm / ml in 0.5M NaPO4 and 7% SDS solution overnight at 65°C. Wash twice a...
Embodiment 2
[0417] Expression of a truncated form of VEGF-2 (SED ID NO: 4) by in vitro transcription and translation
[0418] VEGF-2 cDNA was transcribed and translated in vitro to determine the size of the truncated form of VEGF-2 and the translatable polypeptide partially encoded by VEGF-2 cDNA. Two VEGF-2 inserts in the pBluescript SK vector were PCR amplified with three pairs of primers, 1) M13 reverse and forward primers; 2) M13 reverse primer and VEGF primer F4; and 3) M13 reverse primer and VEGF primer F5. These primer sequences are shown below.
[0419] M13 reverse primer: 5'-ATGCTTCCGGCTCGTATG-3' (SEQ ID NO: 9). This sequence is positioned upstream of the 5' end of the VEGF-2 cDNA insert in the pBluescript vector, as the cDNA it is in the antisense start orientation. A T3 promoter sequence was positioned between the primer and the VEGF-2 cDNA. M13-2 forward primer: 5'GGGTTTTTCCCAGTCACGAC-3' (SEQ ID NO: 10). This sequence was positioned downstream of the 3' end of the VEGF-2 ...
Embodiment 3
[0424] Cloning and expression of VEGF-2 using baculovirus expression system
[0425] With reference to ATCC No.97149, use the PCR oligonucleotide primer corresponding to this gene 5' and 3' sequence to amplify the DNA sequence of the VEGF-2c albumen of 46 amino acids without N terminal:
[0426] The 5' primer sequence is TGT AAT ACG ACT CAC TAT AGG GAT CCCGCC ATG GAG GCC ACG GCT TAT GC (SEQ ID NO: 12) and contains a BamH1 endonuclease site (in bold) and VEGF-25' Sequence complementary 17 nucleotide sequence (nt.150-166).
[0427] The 3' primer has the sequence GATC TCT AGA TTA GCT CAT TTG TGGTCT (SEQ ID NO: 13) and contains a restriction enzyme cleavage site XbaI and 18 nucleotides complementary to the VEGF-23' sequence including a stop codon and a stop codon The 15nt sequence.
[0428] Amplified fragments were separated from 1% agarose gels using a commercially available kit ("Geneclean," BIO101, Inc., La Jolla, CA). This fragment was digested with endonucleases BamH1 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com