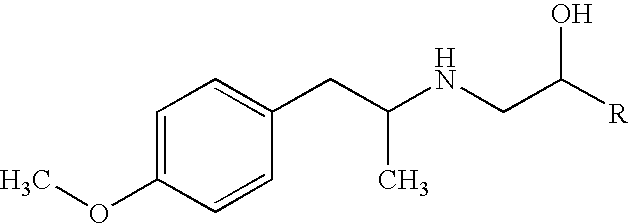
Pharmaceutical solution formulations for pressurised metered dose inhalers
a technology of pressurised metered dose and formulation, which is applied in the directions of drug composition, dispersed delivery, aerosol delivery, etc., can solve the problems of patient still being under treatment, and achieve the effect of improving the co-deposition of said drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
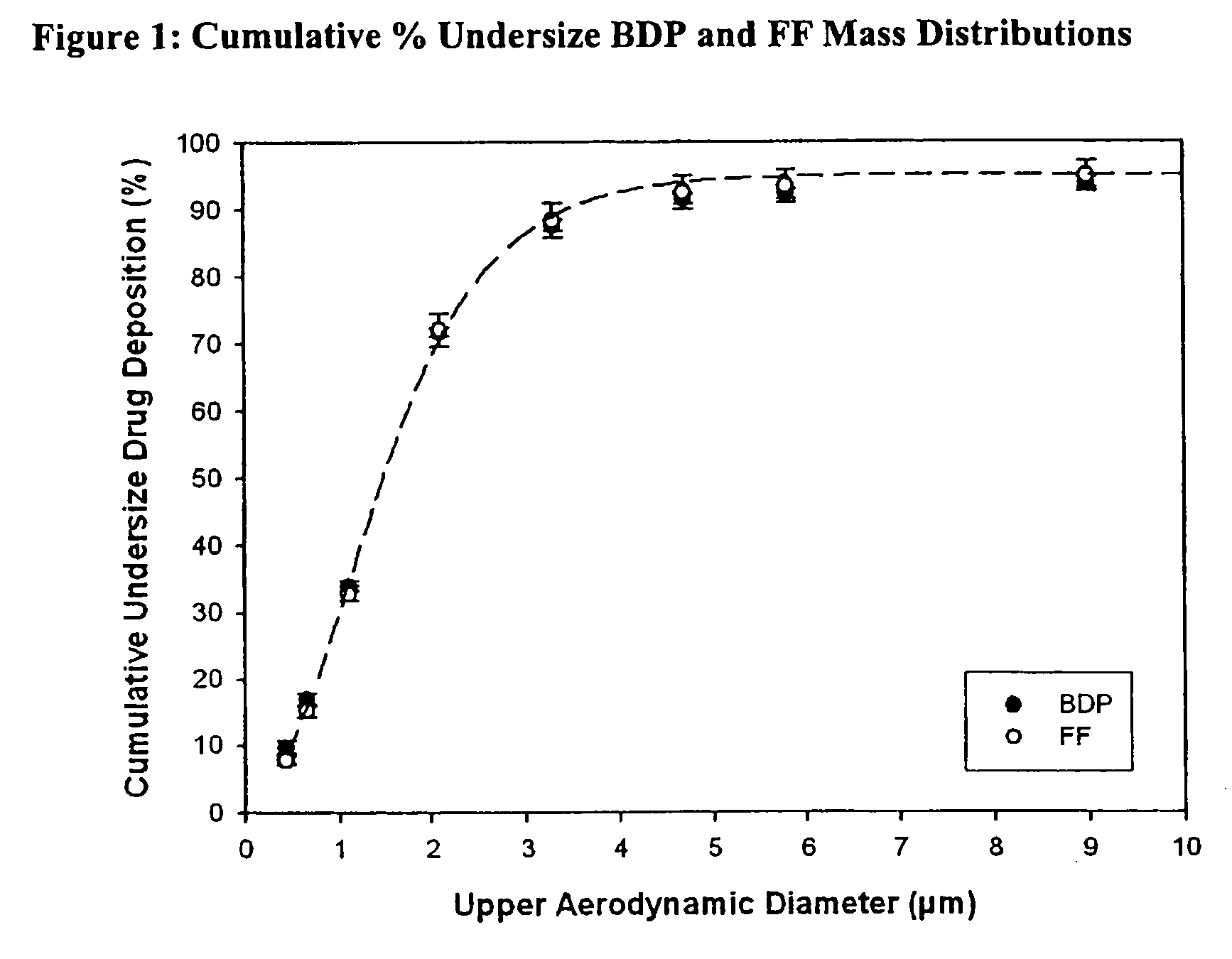
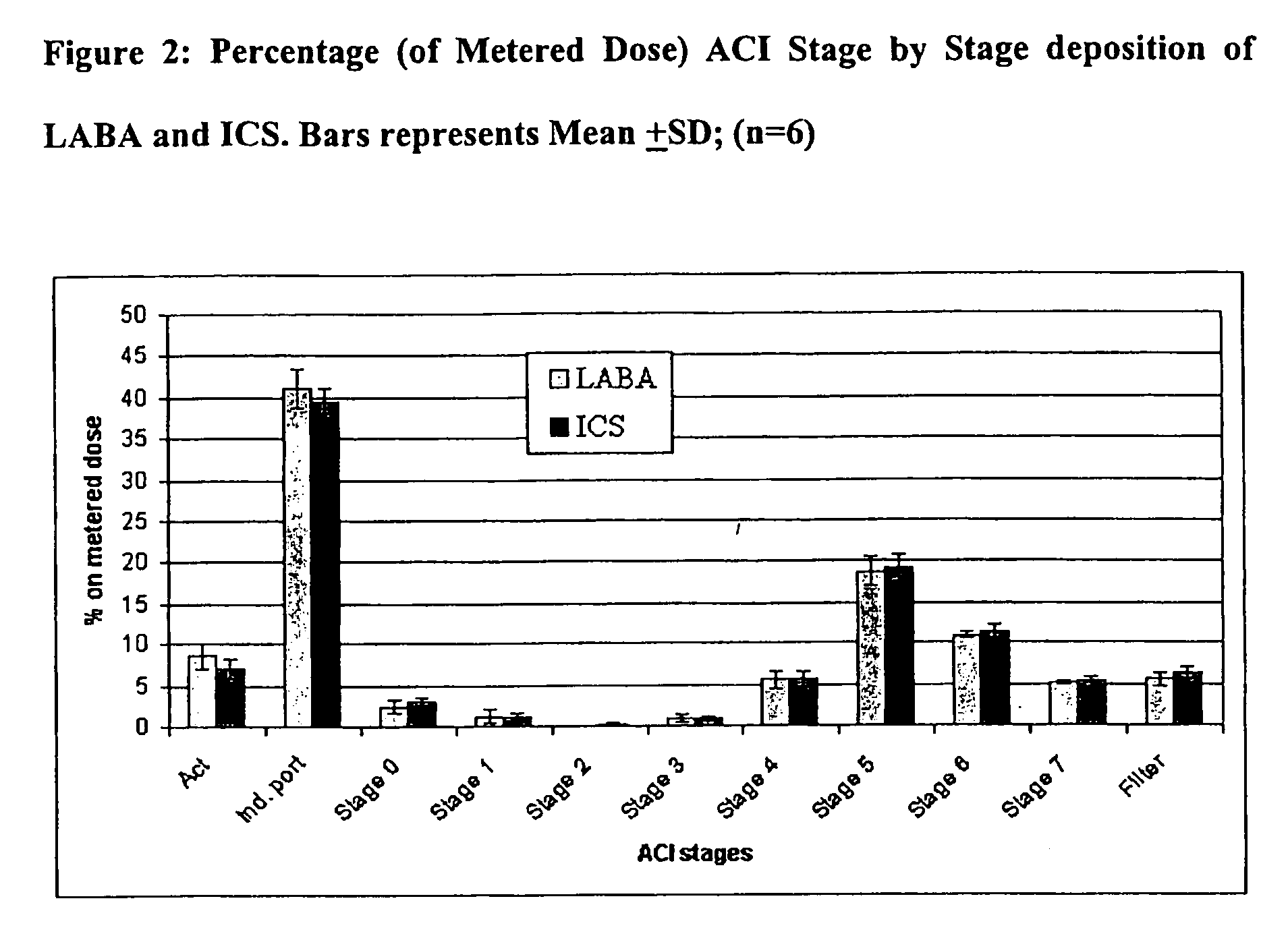
Particle Size Distribution for Formoterol Fumarate and Beclometasone Dipropionate within an Ethanol Based Solution HFA pMDI by Andersen Cascade Impactor (ACI)
[0070]The solution formulation contained beclometasone dipropionate (“BDP”) (100 μg / dose) and formoterol fumarate (“FF”) (6 μg / dose) in 50 μl in HFA 134a propellant vehicle with 12% w / w ethanol as cosolvent and 0.024% w / w hydrochloric acid (1M) as stabilizer. The formulation was packed in cans fitted with 50 μl valves and fired using a 0.30 mm actuator. Aerodynamic particle size assessments were conducted using an Andersen Cascade Impactor fitted with a USP induction port at the beginning and end of can-use life from each of two batches. Each determination was obtained by sampling 15 consecutive doses at a sampling flow rate of 28.3 l / minute. For each can tested the delivered dose was determined using DUSA methodology (Dose Unit Spray Apparatus) at the beginning, middle, and end of can-use life. Quantification of BDP and FF wit...
example 2
Solution Combination Containing Carmoterol Hydrochloride (TA 2005) as LABA and Budesonide (BUD) as ICS
[0073]The LABA TA 2005 is present in the combination at a strength of 1 μg / dose per actuation while budesonide is present at 100 μg / dose per actuation within an acidified ethanol solution pressurized with HFA 134a. Aerodynamic assessment of fine particles was performed by sampling 10 consecutive doses from each pMDI into an ACI. The impactor was fitted with a USP induction port and operated at a sampling flow rate of 28.3 l / minute. Three pMDIs were tested (from the beginning, middle, and end of batch) and were tested at the beginning and end of life. Drug deposition within the impactor was quantified using an HPLC assay. The fine particle dose (FPD) was determined by summation of the drug collected on the ACI stages between S3 and filter.
[0074]Table 3 summarizes the deposition of TA 2005 and budesonide on the individual stages of the ACI, while Table 4 summarizes the aerosol perform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com