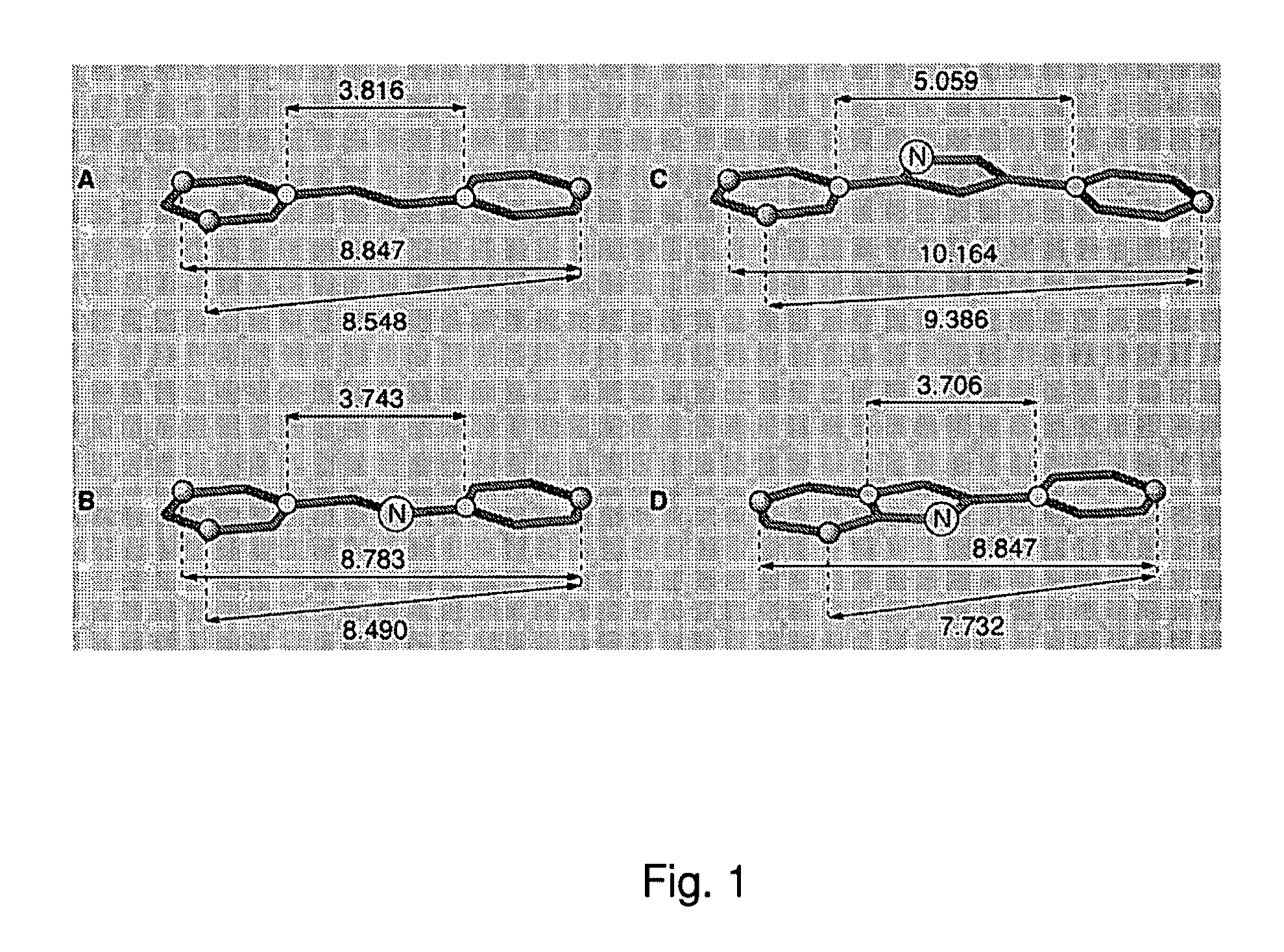
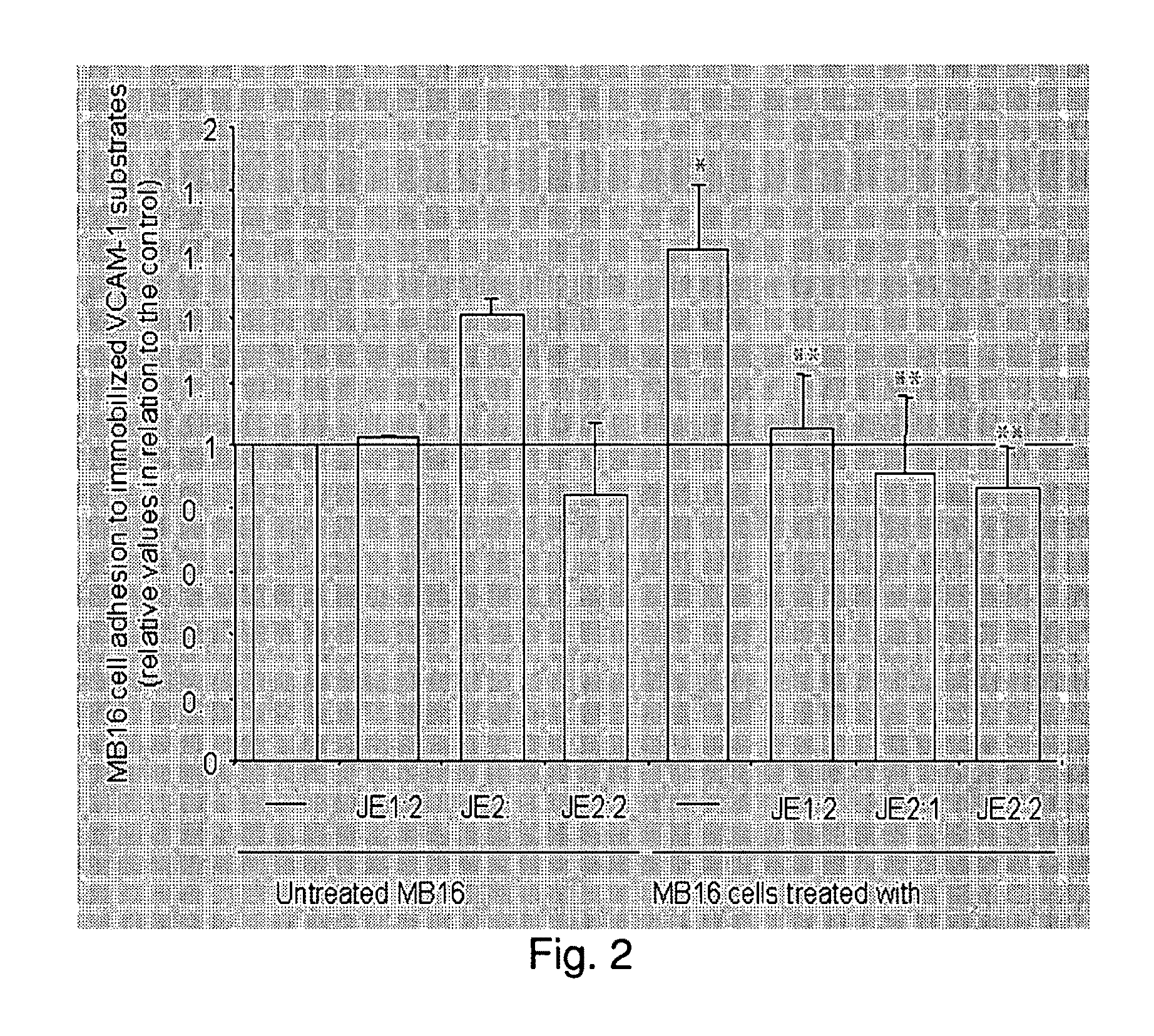
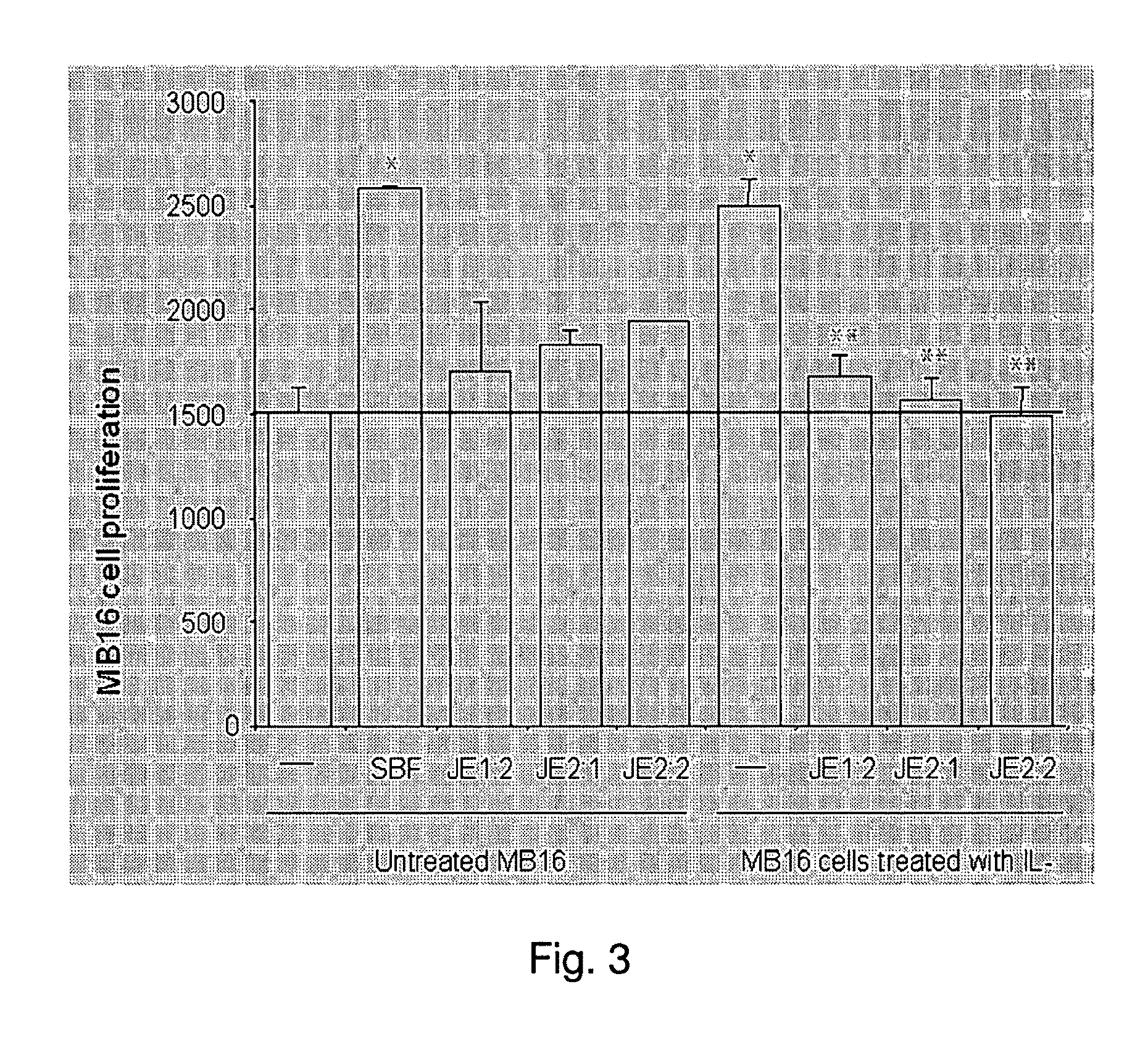
Nitrogenatd trans-stilbene analogs, method for the obtention and medical applications thereof
a technology of transstilbene and nitrogenatd, which is applied in the preparation of isocyanic acid derivatives, drug compositions, biocides, etc., can solve the problem of not being able to achieve the usual high yield of this method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples of embodiment
Example 1
Preparation of 5-((E)-(4-Hydroxyphenylimine)methyl)benzene-1,3-diol, of the Following Structural Formula
[0085]
[0086]Anhydrous magnesium sulfate was added to a suspension of dry dichloromethane (5 ml) of 4-aminophenol (0.109 g, 1 mmol) and of 3,5 dihydroxybenzaldehyde (0.138 g, 1 mmol). The resulting mixture was agitated at ambient temperature for 3 hours. In following, the solvent was evaporated a low pressure and ethanol (5 mL) is added to the resulting residue. The mixture was heating to boiling and then filtered. The filtrate was evaporated under low pressure to yield a residue which was ground to a minimal quantity of cold ethanol, thus yielding 5-((E)-(4-Hydroxyphenylimine)methyl)benzene-1,3-diol. Yield: 100%; p. f. (° C.) 162 (desc.).); IR (KBr) 3494, 3287, 1631, 1598, 1508, 1461, 1339, 1273, 1155 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 9.44 (s, 1H), 9.42 (s, 2H), 8.38 (s, 1H), 7.15 (d, J=7.8 Hz, 2H), 13C NMR (63 MHz, DMSO-d6) δ 158.6, 157.4, 156.2, 142.6, 138.4, 122.5, 115...
example 2
Preparation of 3,5-bis(3,5-dimethoxyphenyl)-1H-pyrrole-2-methyl carboxylate, of the Following Structural Formula
[0087]
and of 3,5-bis(3,5-dimethoxyphenyl)-4-nitro-1H-pyrrole-2-methyl carboxylate, of the following structural formula:
[0088]In a spherical flask, (3,5-dimethoxybenzyllidenamine)methyl acetate (2.0 g, 8.4 mmoles) was dissolved in 84 ml of CH3CN, then adding 1.2 ml (1.25 mmoles) de triethyllamine, 1,3-dimethoxy-5-(2-nitrovinyl)benzene (1.8 g, 8.4 mmoles) and 0.21 g (1.25 mmoles) of AgOAc. The progress of the reaction was monitored by means of thin-layer chromatography. Following completion of the reaction (approx. 5 hours), the mixture was filtered through a celite bed and the filtrate washed with an aqueous NH4Cl solution (2×84 ml) and H2O (2×84 ml), was then dried on anhydrous Na2SO4 and evaporated at low pressure. The crude portion was purified by means of pressurized chromatography column (AcOEt / Hx). 1.8 g (4 mmoles) of the oil obtained was dissolved in 40 ml of 2-metho...
example 3
Preparation of 5-(3,5-dimethoxyphenyl)-3-(4-methoxyphenyl)-1H-pyrrole-2-methyl carboxylate, of the Following Structural Formula
[0091]
and of 5-(3,5-dimethoxyphenyl)-3-(4-methoxyphenyl)-4-nitro-1H-pyrrole-2-methyl carboxylate, of the following structural formula:
[0092]Both compounds were prepared and separated by means of a procedure similar to that described in Example 2 hereinabove.
[0093]5-(3,5-dimethoxyphenyl)-3-(4-methoxyphenyl)-4-nitro-1H-pyrrole-2-methyl carboxylate: Yield: 20%; p.f. 194-145° C.; IR 3266, 1683, 1597, 1507, 1276, 1211, 1166 cm−1; 1H-RMN (δ ppm. CDCl3) 9.42 (s. 1H). 7.32 (d. 2H. J=8.3 Hz). 6.96 (d. 2H. J=8.3 Hz). 6.71 (s. 2H). 6.58 (s. 1H). 3.87 (s. 3H). 3.84 (s. 3H). 3.71 (s. 3H); 13C-RMN (δ ppm. CDCl3) 161.4, 161.2, 159.7, 134.4, 133.9, 131.4, 130.6, 127.6, 123.2, 118.3, 113.5, 107.2, 103.5, 102.4, 55.9, 55.5, 52.4. Calc. Analysis for C21H20N2O7: C. 61.16; H. 4.90; N. 6.80. Found: C. 61.47; H. 4.92; N. 7.00%.
[0094]5-(3,5-dimethoxyphenyl)-3-(4-methoxyphenyl)-1H-p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com