Methods of treating multiple myeloma and myeloma-induced bone resorption using integrin antagonists
a technology of integrin antagonists and multiple myeloma, which is applied in the field of multiple myeloma treatment, can solve the problems of inconclusive studies, inconclusive reports of myeloma cell factors, and pain in the bone, so as to improve the quality of life of patients and stop progressive bone destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
5TGM1 Myeloma Cells
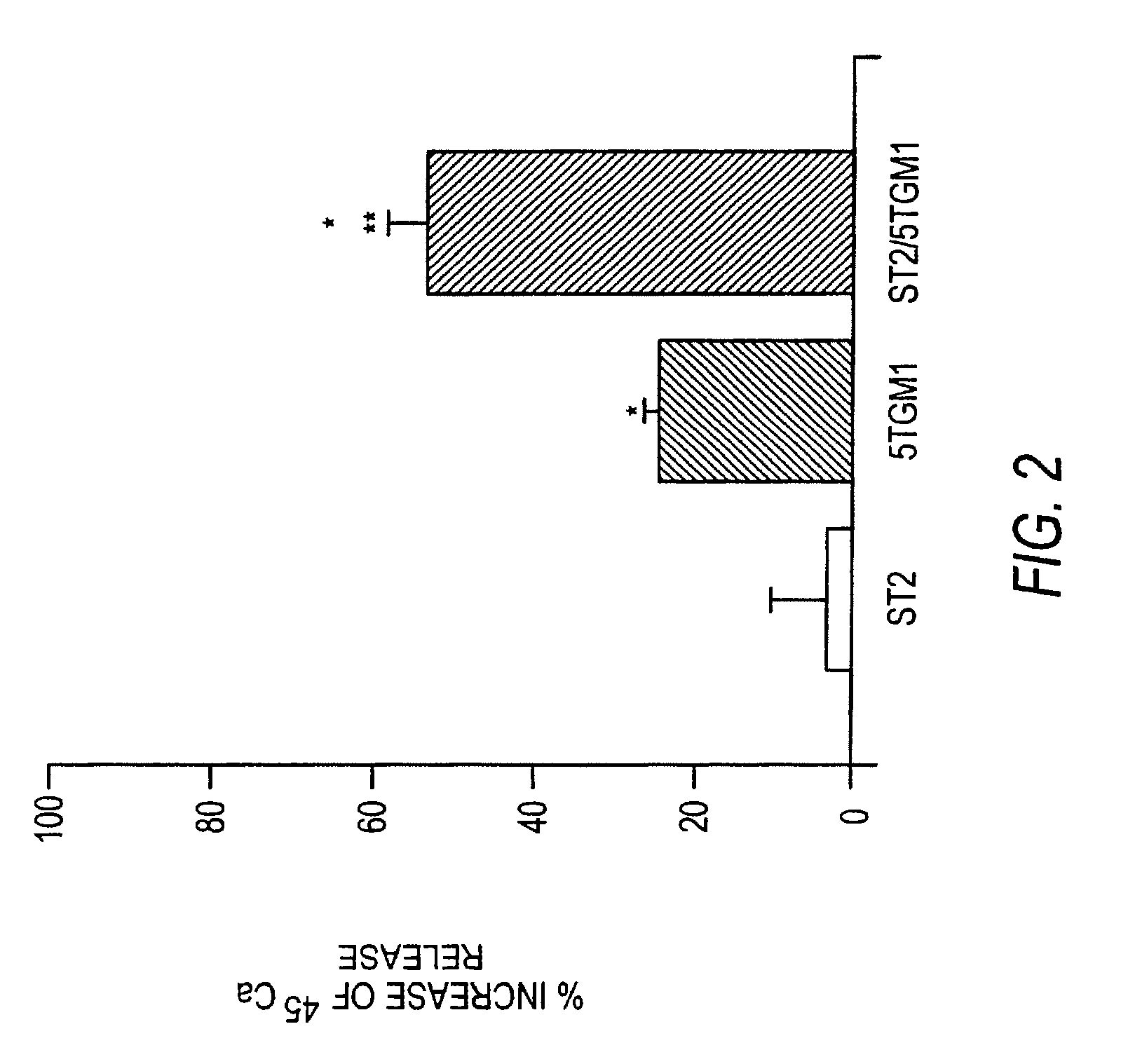
[0159]5TGM1 myeloma cells were initially derived from a myeloma which arose spontaneously in aged C57BL / KaLwRij mice (Garrett 1997, Vanderkerken 1997). Cells were grown in Isocove's Modified Dulbecco's Medium (IMDM, Life Technologies Inc., Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS, Summit, Fort Collins, Colo.) and 1% penicillin-streptomycin solution (GIBCO, Grand Island, N.Y.) at 37 C in 5% CO2 atmosphere. For in vitro experimentation described below, 5TGM1 cells between passage 25 and 30 were used.
Antibodies, Soluble VCAM-1
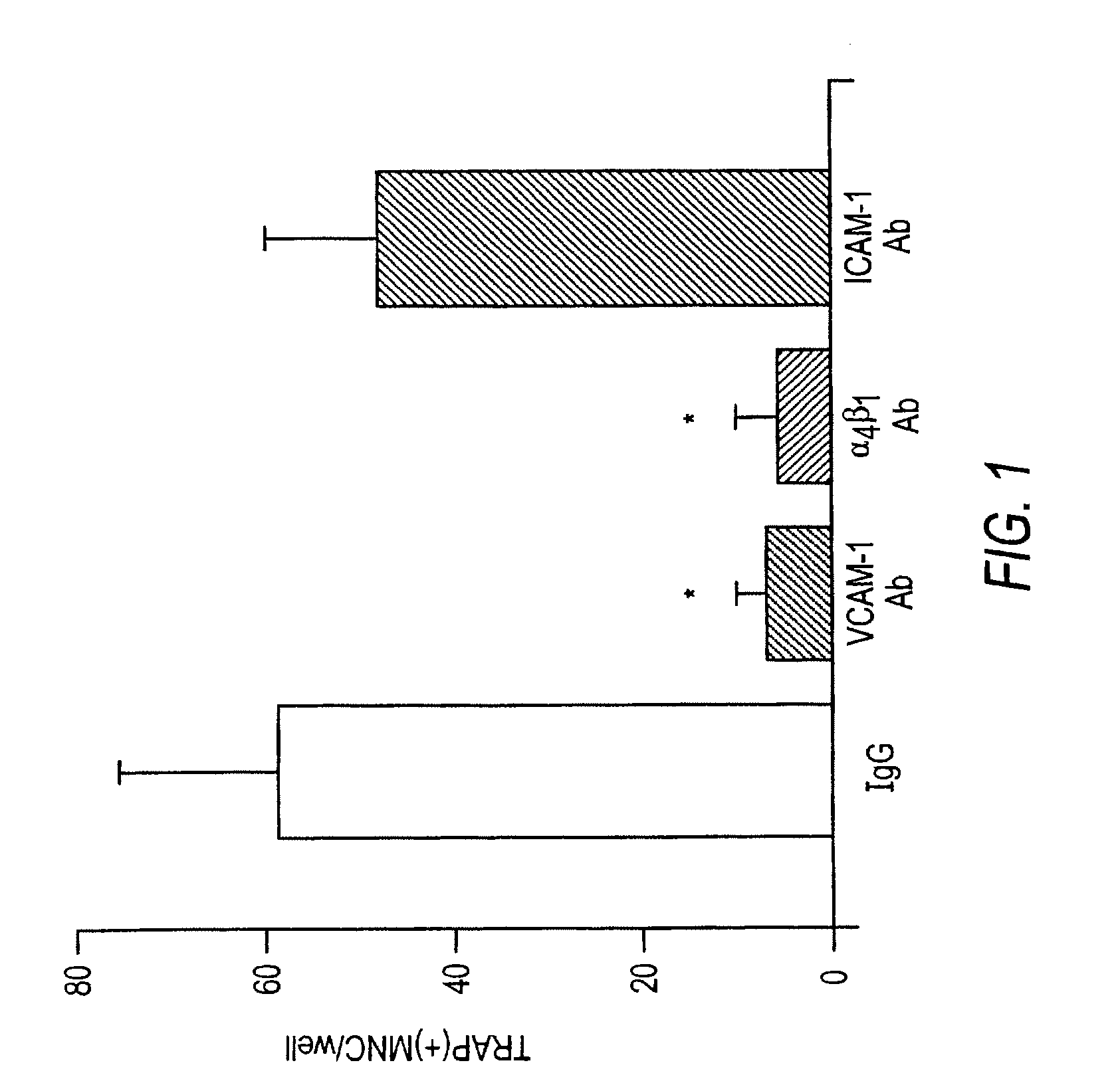
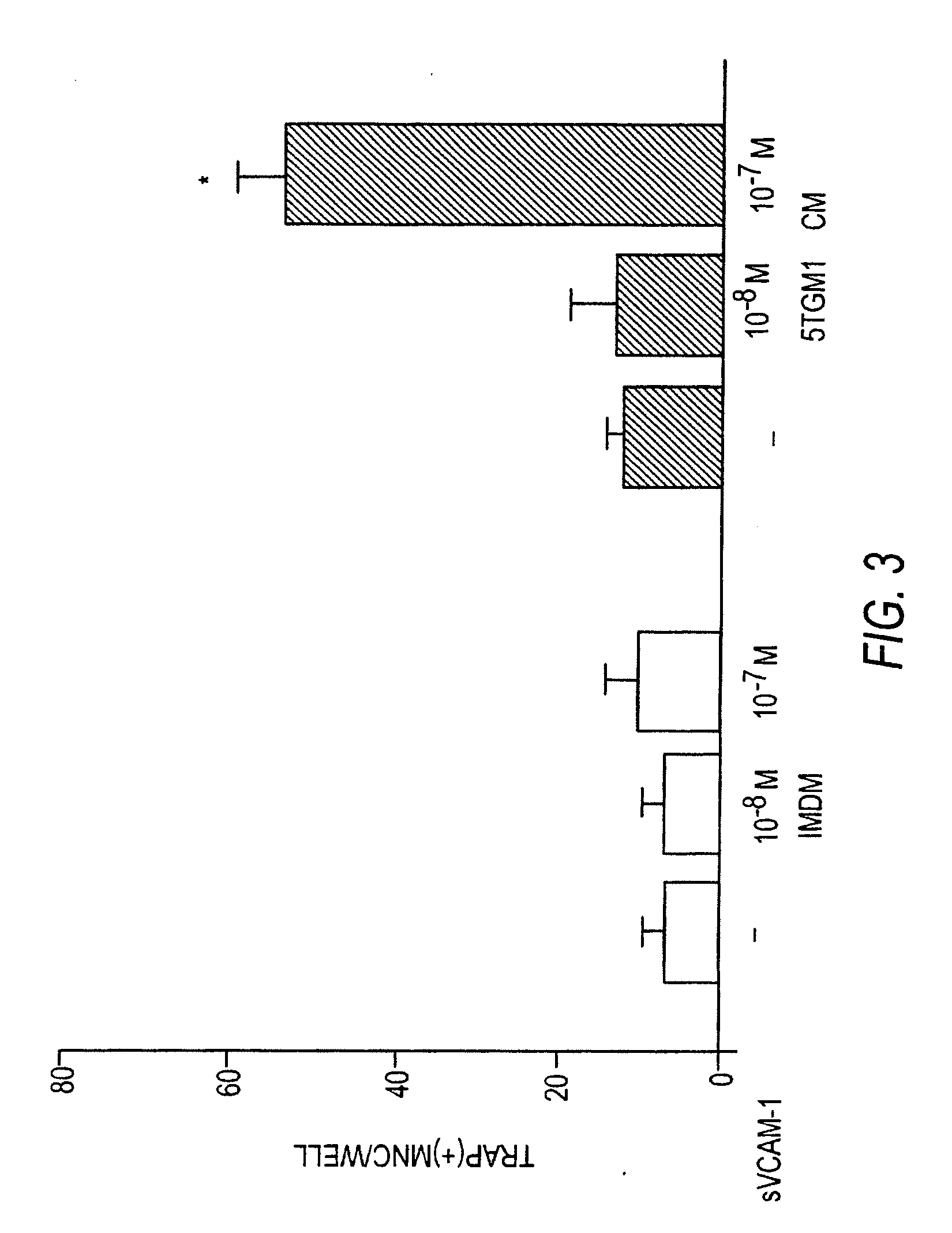
[0160]Neutralizing antibodies against murine VCAM-1 (M / K-2.7), integrin VLA-4 (PS / 2 mAb), and Intercellular Adhesion Molecule-1 (ICAM-1, YN1 / 1.7), were kindly gifted by Dr. Kensuke Miyake (Saga Medical University, Saga, Japan). Recombinant soluble VCAM-1 (Lobb et al., 1991), containing the 7 extracellular domains of human VCAM-1, was the gift of Dr. Roy Lobb, Biogen Inc., Cambridge, Mass.
Reverse Trans...
example 2
In Vivo Experiments
[0179]Our in vitro studies suggest that the interaction between VLA-4 on myeloma cells with VCAM-1 on marrow stromal cells may play a key role in the induction of bone resorbing activity by myeloma. We have taken the key step of testing this hypothesis in vivo in an animal model which accurately reflects human disease.
[0180]A. In this experiment, mice were injected with 1 e 5 5TGM1 myeloma cells, which were allowed to colonize the bone marrow. Mice were split into two groups of three, one serving as a control group, and the second treated biweekly beginning on day 8 with mAb PS / 2. Levels of IgG2b, the antibody isotype produced by 5TGM1 myeloma cells, were measured weekly from weeks 1 to 6. Treatment with mAb at a dose of 80 μg per injection (˜4 mg / kg) biweekly strongly inhibited IgG2b production, indicative of significant inhibition of myeloma cell survival and growth in vivo (FIG. 4). Further, the treated mice showed reduced incidence of paraplegia (all 3 untreat...
example 3
Other In Vivo Experiments
[0186]Based on the information presented herein for the first time, persons having ordinary skill in the art can readily confirm and extend the importance of the α4 integrins and their ligands in multiple myeloma using the murine animal model described.
[0187]The following series of experiments are well within the level of skill in the art based upon the present disclosure but serve merely to exemplify, and not limit, the types of work.[0188]1) Dose response to mAb PS / 2 to determine the optimal biweekly maintenance dose. 80 μg shows good efficacy, but 40 μg was without effect. One examines higher doses up to 20 mg / kg two or three times weekly to determine optimal dosing.[0189]2) Patients present with disease at different stages of severity, linked to increased tumor burden. One examines the efficacy of mAb PS / 2 given at different times after establishment of disease, i.e., one compares treatment initiation at 8 days (see for example FIG. 4) to initiation afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com