Nucleotide vector, composition containing such vector, and vaccine for immunization against hepatitis
a technology of nucleotide vectors and compositions, applied in the direction of immunological disorders, antibody medical ingredients, peptide sources, etc., can solve the problems of time-consuming and expensive production and maintenance of these vaccines, widespread and serious international health problems, and inability to mass immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Induction of Antibodies Against a Hepatitis B Surface Antigen by Sequential Injection of Bupivacaine and of a Plasmid Carrying a Gene Coding for the Antigen
[0094](1) Materials and Methods
[0095]1.1 Bupivacaine Pretreatment
[0096]All experiments were made on the muscles of the anterior tibia (AT) of mice C57BL / 6 aged between 5 to 7 weeks.
[0097]A single degeneration-regeneration cycle of the muscle fibers is induced in the muscles of the anterior tibia of non-anaesthetized mice, by intramuscular injection of 50 μl marcaine (bupivacaine 0.5%, DMSO 1%) sold by Laboratoires Astra, France. The solution is injected using a tuberculosis syringe with a needle fitted into a polyethylene sleeve in order to limit the penetration depth to 2 mm.
[0098]As marcaine is an anesthetic, injections into the right and left legs were performed at 10 to 30 minute intervals to prevent an overdose.
[0099]1.2 DNA Preparation
[0100]The plasmid used was constructed by cloning into a modified pBlueScript vector of th...
example 2
Comparison of the Efficiency of a Plasmid Injection in the Presence and Absence of Lipids
[0116]A dose of 10 μg plasmid DNA from the SV40-luciferase vector available commercially (“pGL2-Control Vector” from Promega, reference E1 11) in 50 μl of physiological solution was injected into the sucrose pretreated muscle following the method of Davis et al. (Hum. Gene Ther. 4:151-159 (1993)). The injected DNA is mixed earlier with lipids such as dioctadecylamidoglycyl spermine (DOGS) or the following mixtures: DOGS+spermidine, and DOGS+polyethyleneglycol (PEG). The luciferase activity was determined 5 days after the injection.
[0117]These results are shown in table II below.
[0118]They show that the presence of lipids (DOGS) very significantly reduces the efficiency of the plasmid injection with respect to a composition with no lipids (Control).
example 3
Comparison of the Responses of Mice and Rabbits to Plasmids Carrying Different Promoters and Envelope Genes for the HBV Virus
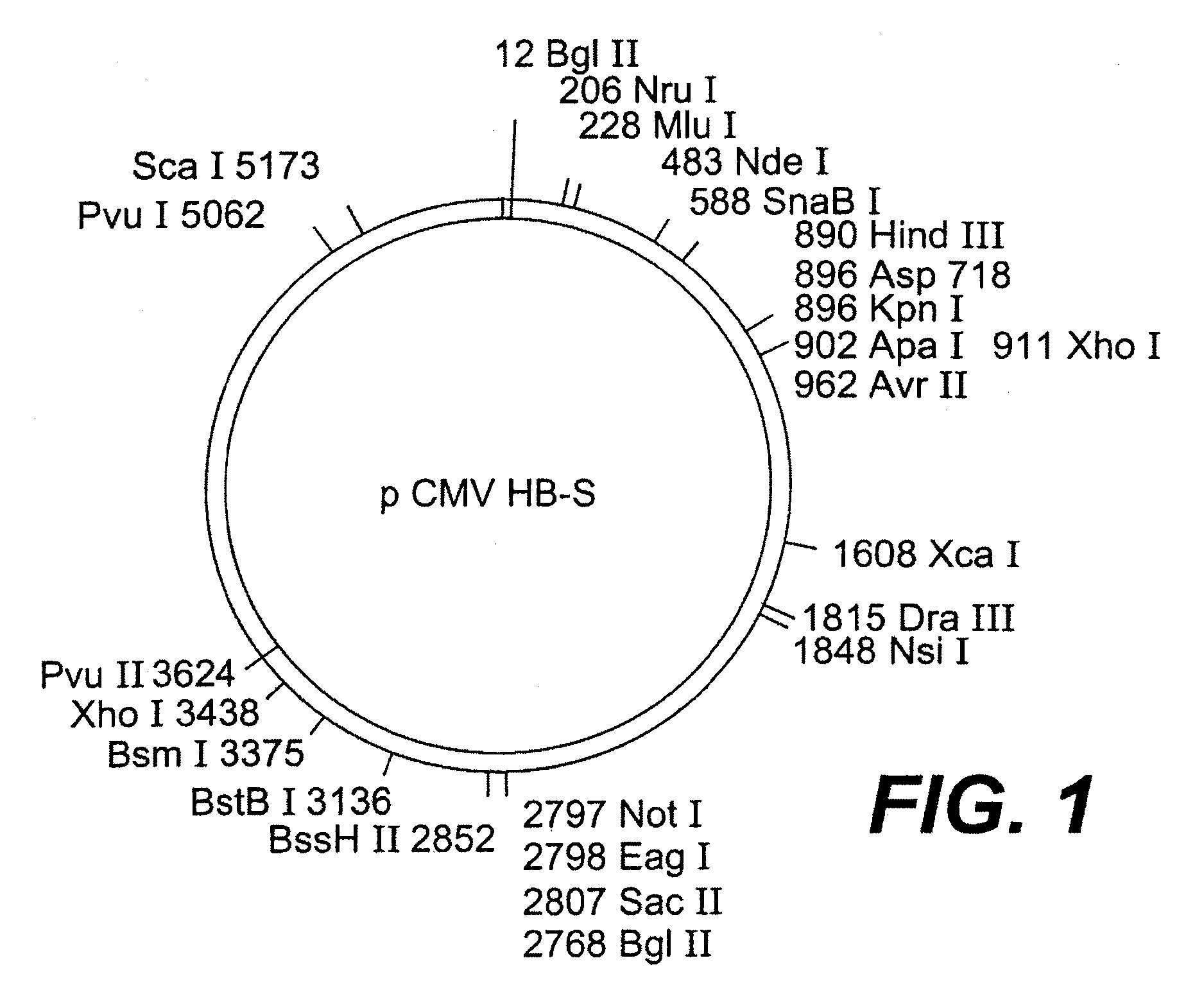
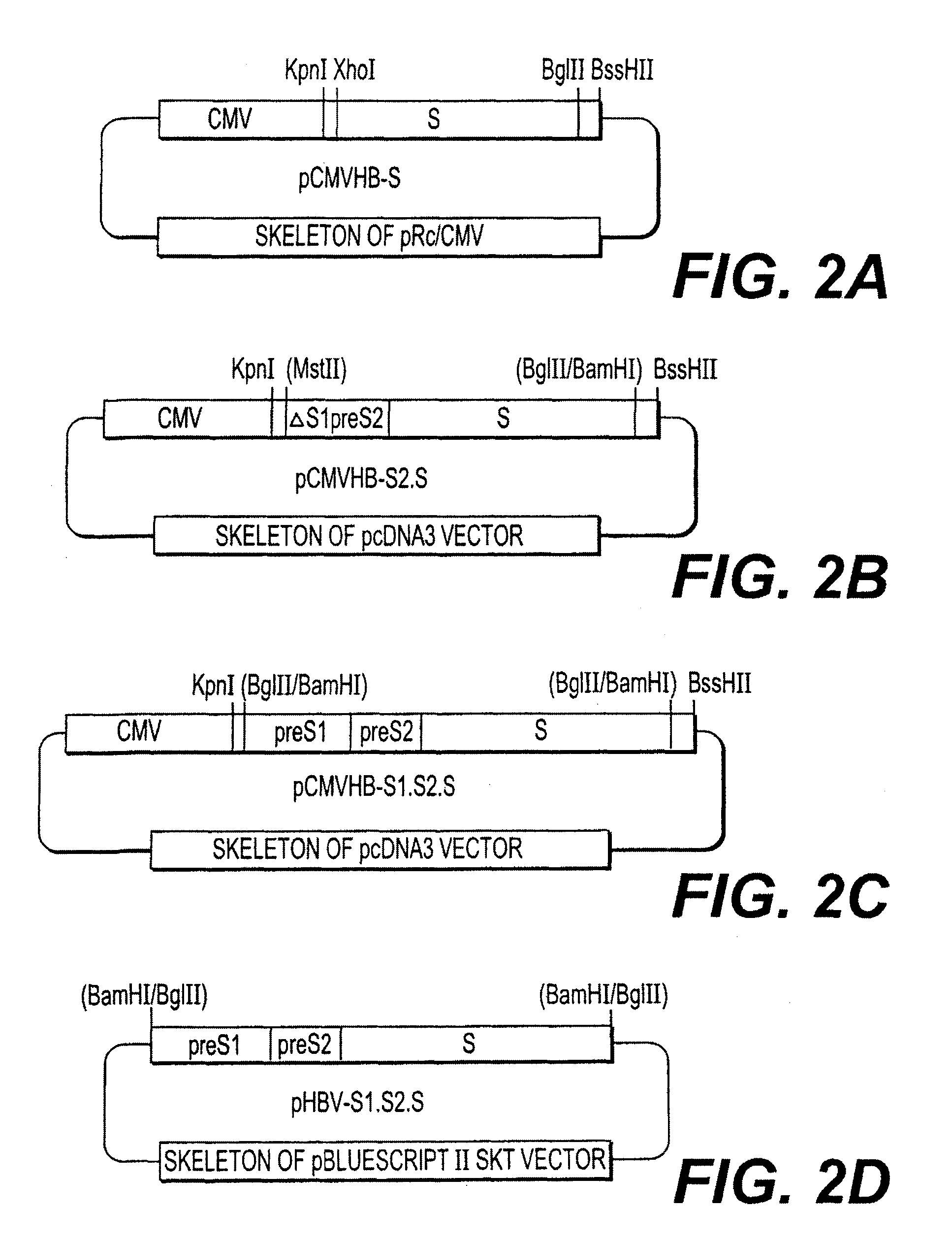
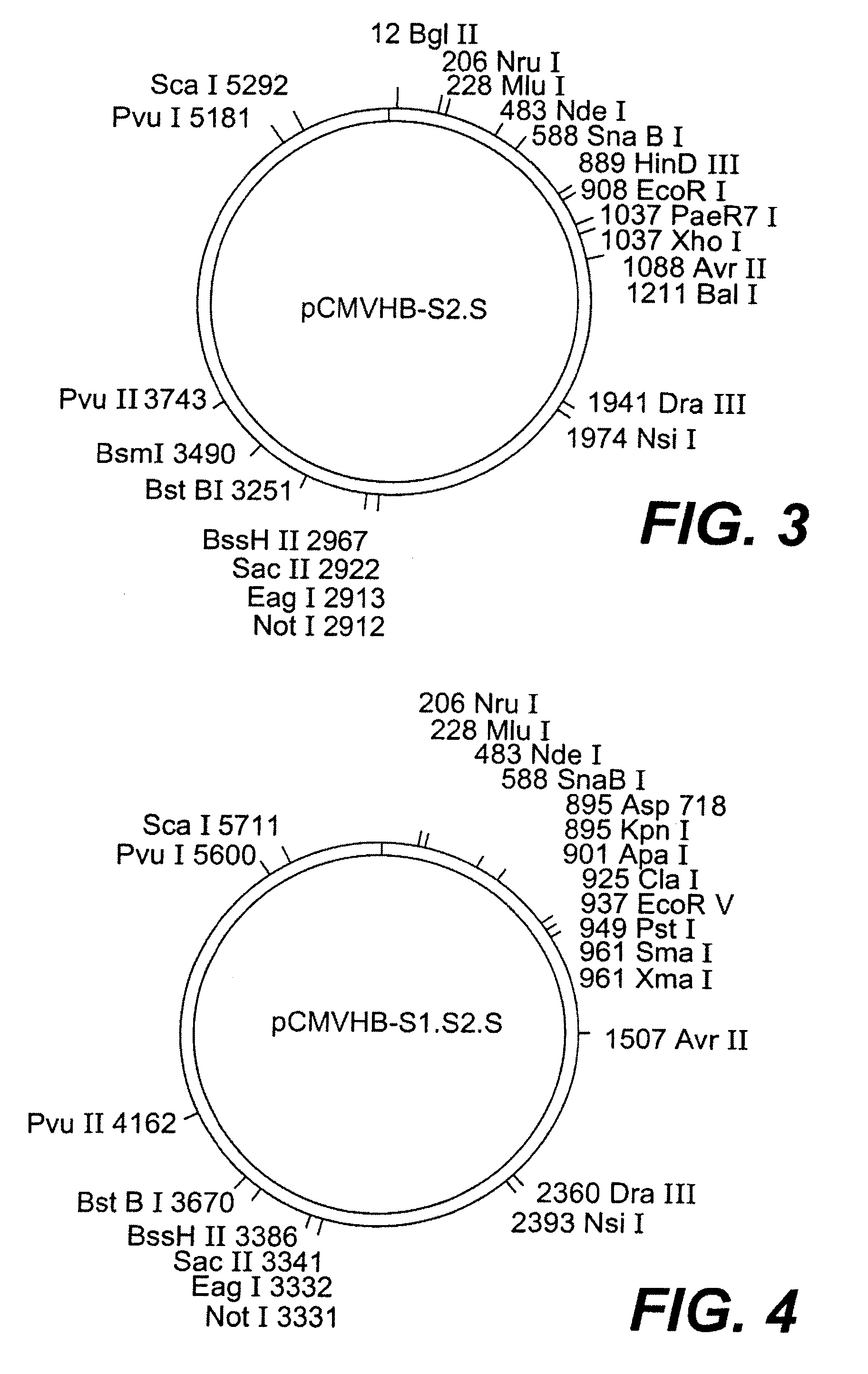
[0119]Five plasmids were constructed allowing the expression of one, two, or three envelope proteins for the HBV virus. In three of the constructions (pCMVHB-S, pCMVHB-S2.S, pCMVHB-S1.S2.S) the genes coding for the HBV virus envelope proteins are put under transcriptional control of the promoter of the CMV virus precursor genes (FIG. 1, FIG. 2A to 2C, FIGS. 3 and 4). The fourth plasmid (pHBV-S1.S2.S) uses the promoter for the HBV virus surface genes contained in the pre-S1 region of this virus (Cattaneo et al. (1983) Nature, 305, 336) (FIG. 2D) as a transcriptional controlling element. In another plasmid, pSVS, the three envelope proteins were placed under control of the SV40 promoter (pSVS) (Michel et al., Proc. Natl. Acad. Sci. USA, 81:7708-7712 (1984)). The construction of the pSVS plasmid is further described in EP0 156 712 B1. In the five constructions, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| penetration depth | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com