Methods and compositions for delivery of exogenous factors to nervious system sites
a technology of exogenous factors and nervious system, which is applied in the direction of drug compositions, peptide sources, peptide/protein ingredients, etc., can solve the problems of limiting functional recovery, lacking the technology to successfully direct stem cell differentiation into the appropriate or desired cell type in vivo, and transplanted neural stem cells (nsc) not differentiate into the appropriate cell type for neuron regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
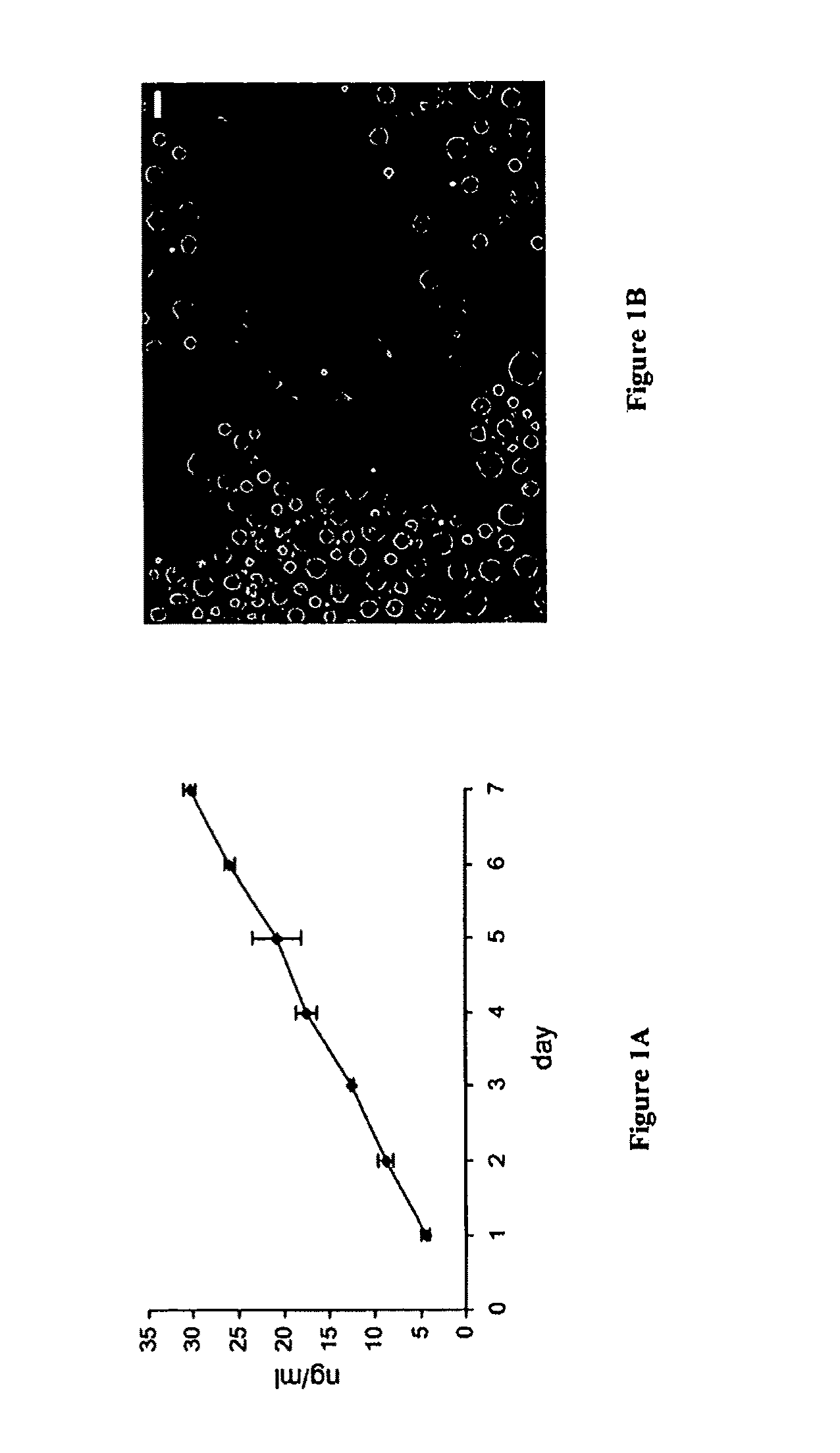
[0184]Shh-Containing Microspheres Release Active Shh Protein In Vitro and are not Toxic to Neural Stem Cells.
[0185]To establish a source of continuous release of Shh, biodegradable microspheres (10-40 μm in diameter) of poly(lactide-co-glycolide) (PLGA) that incorporated 0.5% of Shh as described above (Cat. No. 1314-SH / CF, recombinant human Shh from R&D Systems, Minneapolis, Minn., corresponding to SEQ ID NO:2, the active N-terminal Shh fragment, which corresponds to Cys24-Gly 197 of SEQ ID NO:1) were generated. Microsphere preparation is described above and adapted from the methods of (Fu et al. 2003). To determine the release kinetics 0.1 mg of Shh-containing microspheres or microspheres with PLGA alone (blank sample) were resuspended in 1 ml PBS. 100 μl aliquots were taken out each day and analyzed for the Shh release by ELISA, as shown in FIG. 1A. FIGS. 1A-B show biodegradable PLGA-based microspheres release active Shh in a course of at least 7 days. These microspheres are not t...
example 2
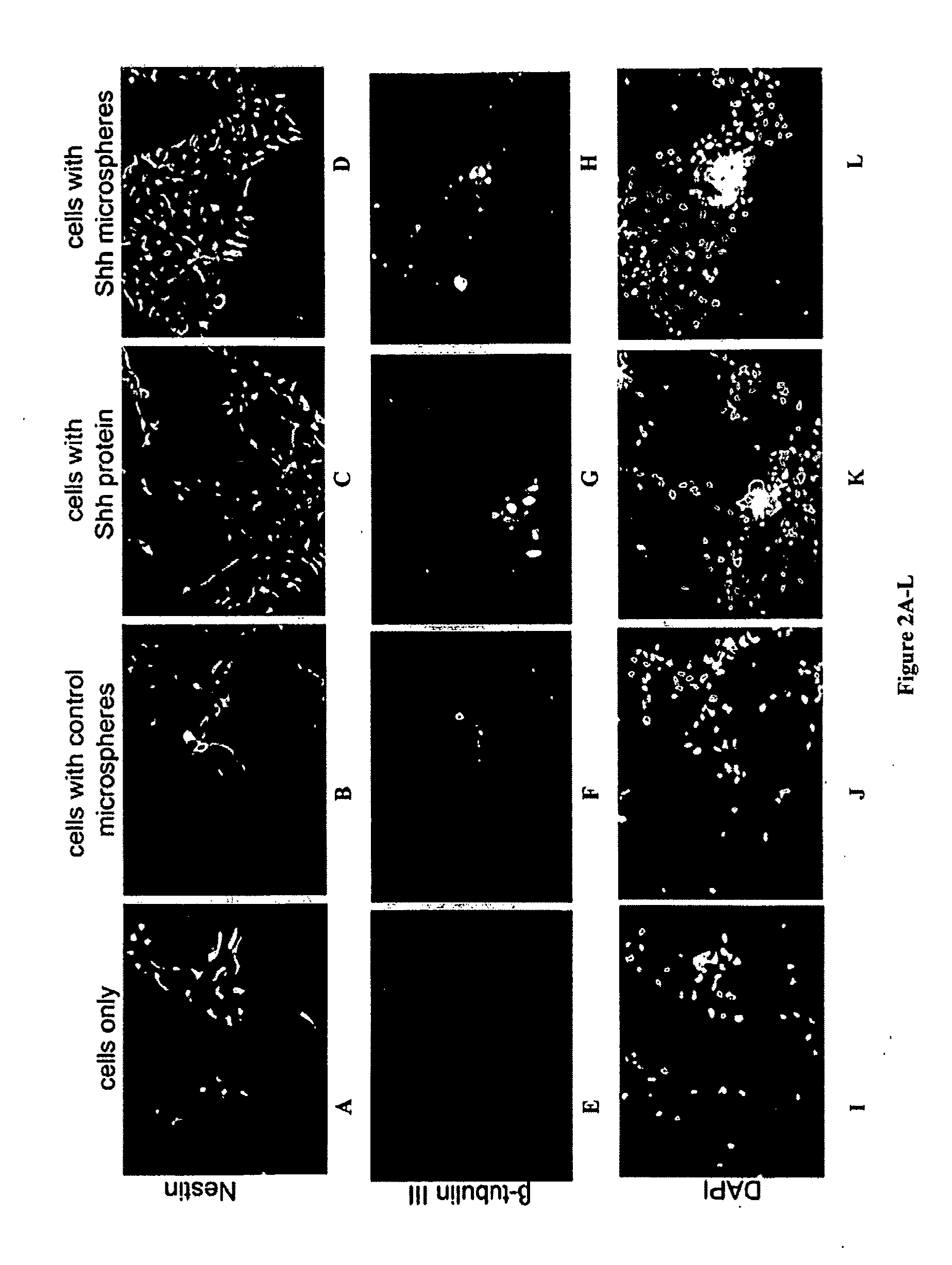
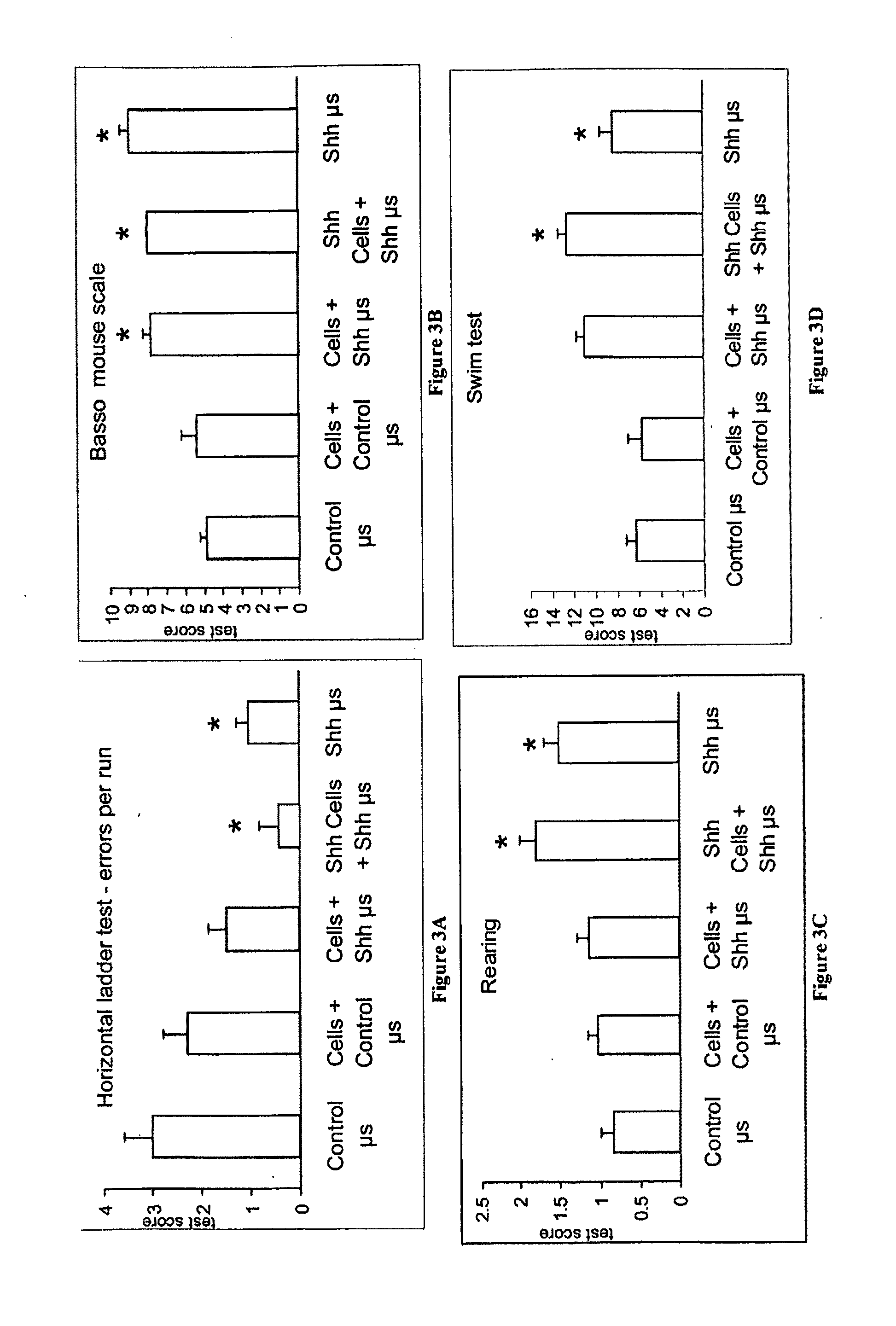
[0189]Transplantation of Shh-Releasing Microspheres or a Combination of Shh-Treated Spinal Cord Neural Stem Cells with Shh-Releasing Microspheres Produced / Resulted in Motor Recovery.
[0190]Shh-releasing microspheres and a combination of Shh-releasing microspheres and endothelial-expanded Shh treated cells were examined as treatments for SCI. To generate endothelial-expanded spinal cord stem cells, E9 mouse spinal cord stem cells were co-cultured with BPAE cells in serum-free medium with or without the addition of 1 μM Shh and 1 μM retinoic acid for 6 days, then removed and injected into adult (10-12 weeks old) mice recipients that had a dorsal over-hemisection spinal cord injury.
[0191]Adult mice were anaesthetized and the dorsal surface of the cord was exposed at T8-9, and the cord was cut down to a depth of 1 mm, representing halfway through the cord from the dorsal surface. This procedure severs the descending corticospinal and ascending sensory spinal axons located in the dorsal c...
example 3
[0194]Injection of Shh-Releasing Microspheres into SCI Resulted in a Reduced Astroglial Scar Formation Compared to Injection of Control Microspheres into SCI.
[0195]After behavioral testing, the mice were sacrificed (four weeks after SCI) and the spinal cords were examined by staining longitudinal sections of the SCI site with GFAP, which is an astrocyte marker (as shown in FIG. 4A). In FIGS. 4A-B, the spinal cord cells appear as bright regions, since they are isolated from GFP transgenic mice and constitutively express green fluorescent protein (GFP). Transplantation of Shh-releasing microspheres resulted in a decrease of the astrocytic scar at the site of injury, as shown in FIG. 4B, demonstrated by reduced staining of GFAP in FIG. 4B (few bright-staining cells on sections treated with Shh-releasing microspheres) compared to FIG. 4A (control microspheres lacking Shh and containing more brighter staining regions). These data illustrate that injection of Shh-releasing microspheres in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com