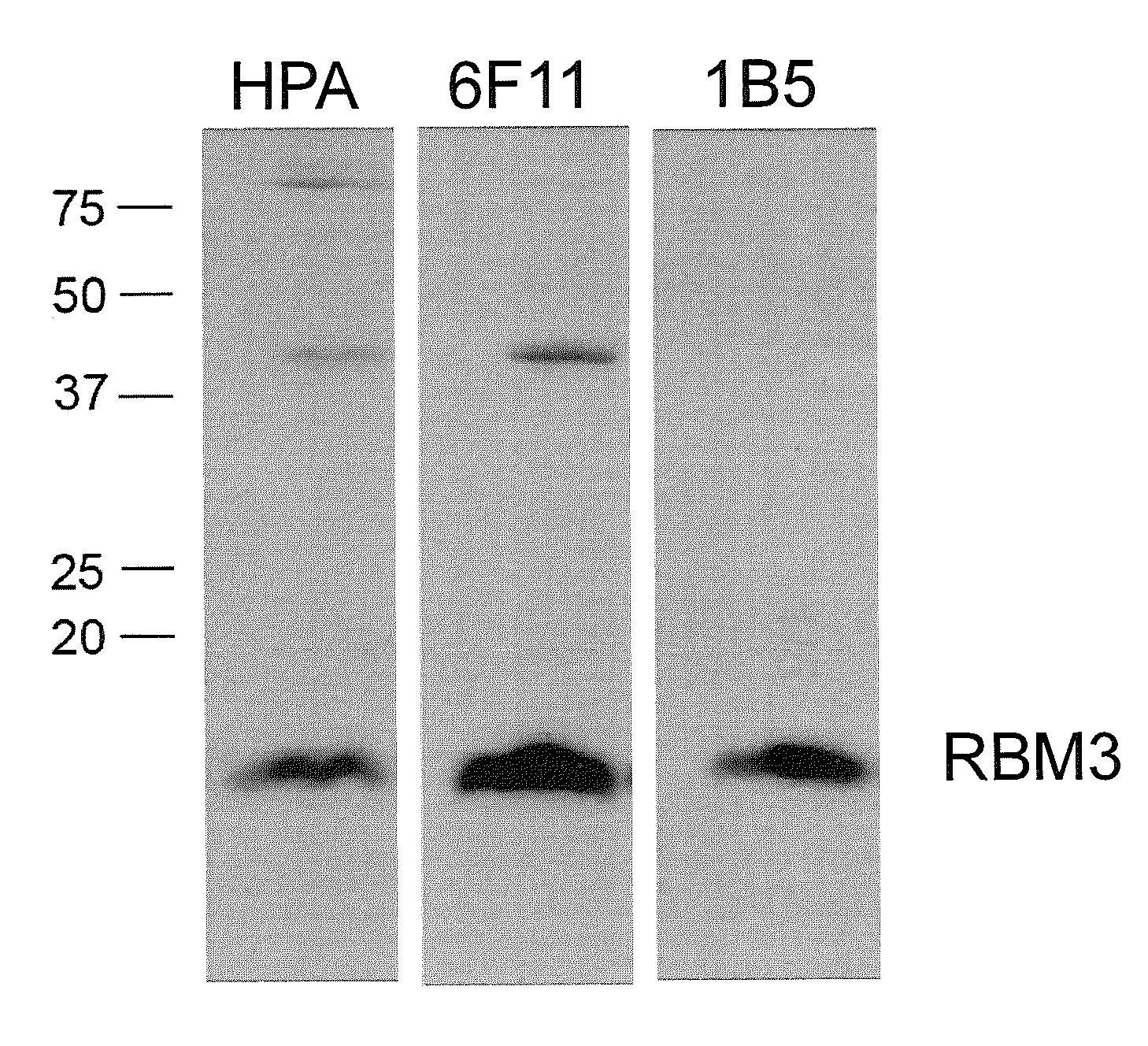
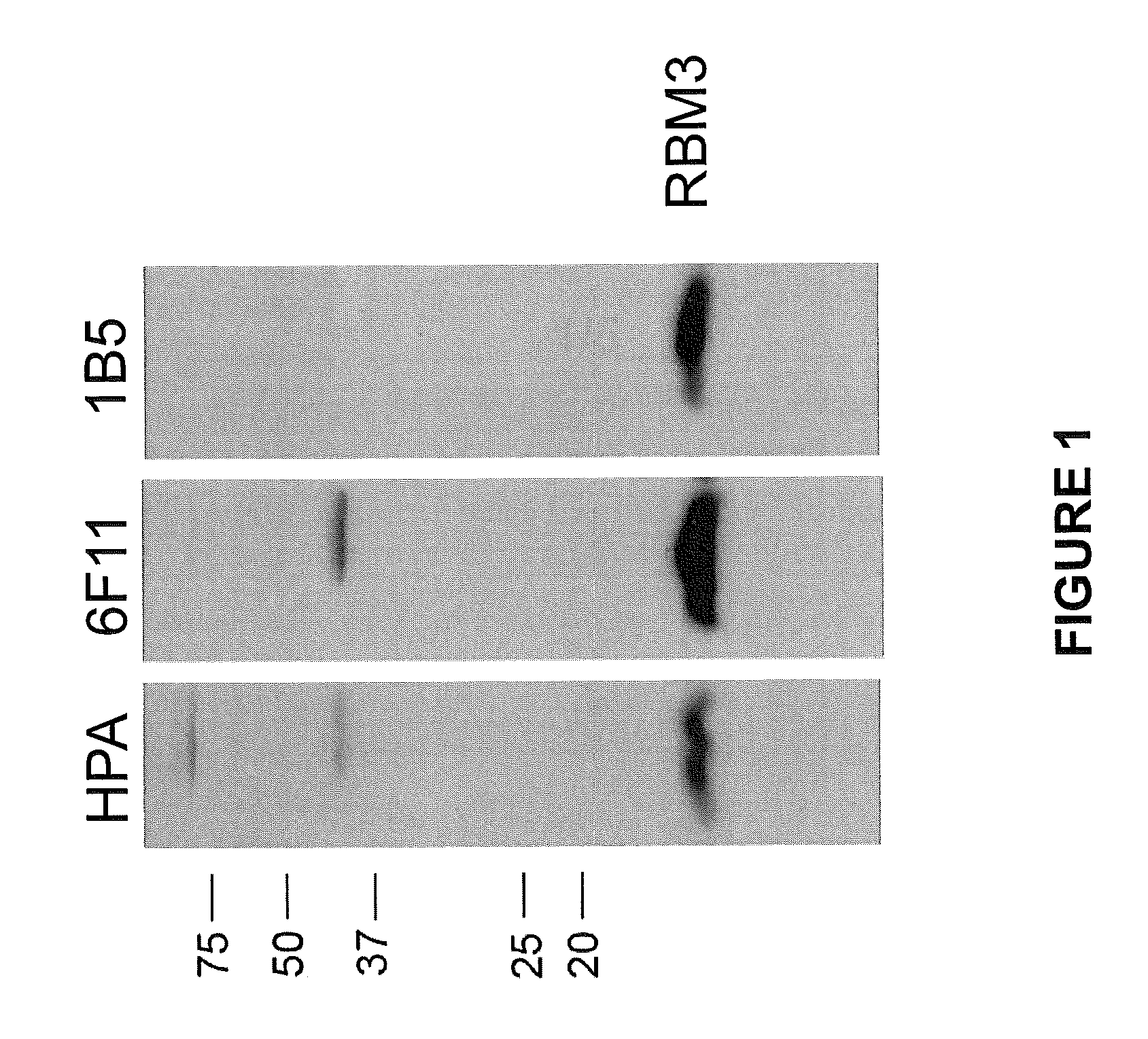
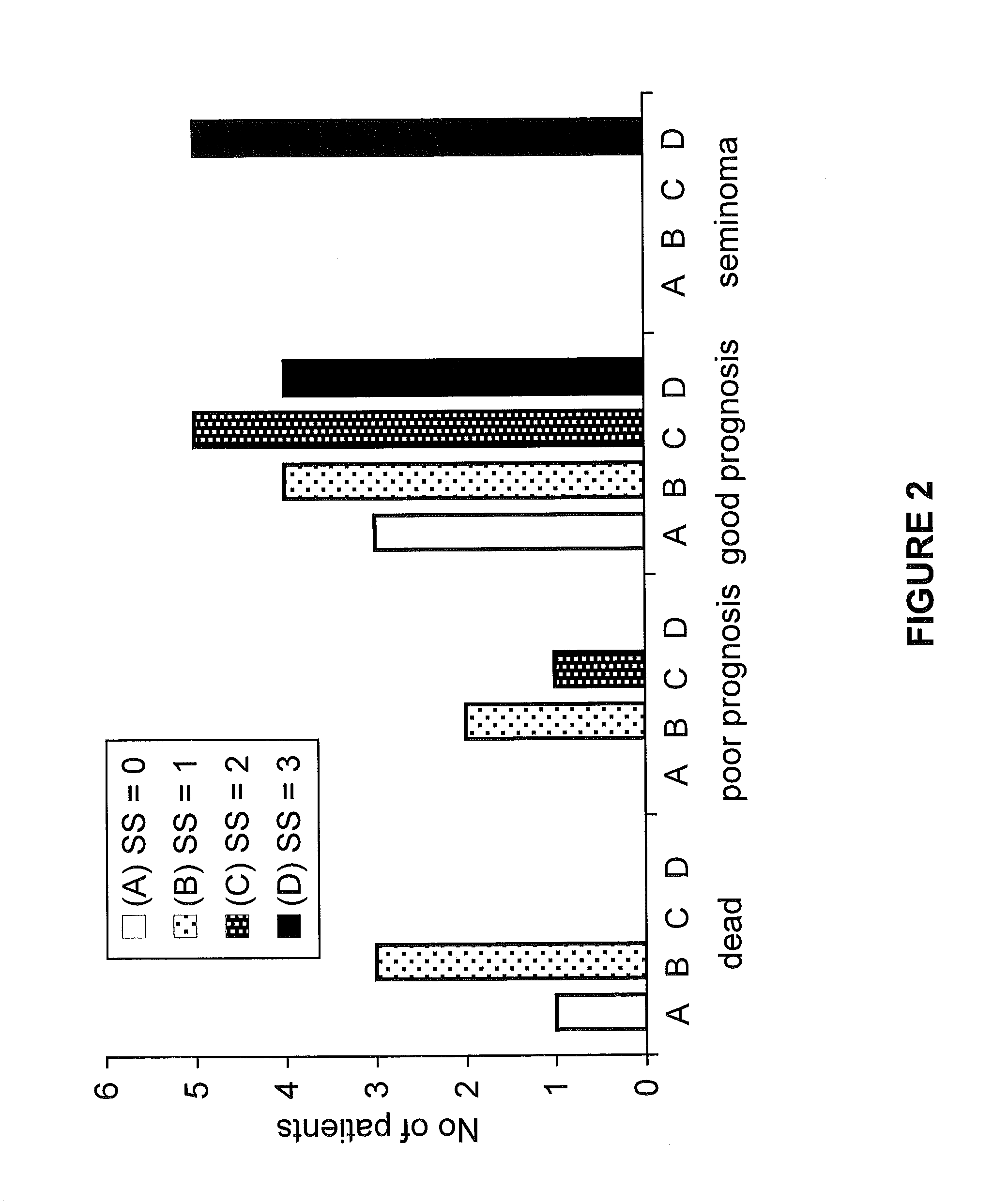
RBM3 in Testicular Cancer Diagnostics and Prognostics
a testicular cancer and diagnostic technology, applied in the field of testicular cancer, can solve the problems of evaluating and standardized, unable to define cancer entirely, and formation of cancer following precancerous proliferation stages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Mono-Specific Polyclonal Antibodies
1. Generation of Antigen
a) Materials and Methods
[0414]A suitable fragment of the target protein encoded by the EnsEMBL Gene ID ENSG00000102317 was selected using bioinformatic tools with the human genome sequence as template (Lindskog M et al (2005) Biotechniques 38:723-727, EnsEMBL, www.ensembl.org). The fragment was used as template for the production of a 134 amino acid long fragment corresponding to amino acids 18-151 (SEQ ID NO:1) of the RBM3 protein (SEQ ID NO:2; EnsEMBL entry no. ENSP00000365946).
[0415]A fragment of the RBM3 gene transcript containing nucleotides 281-682, of EnsEMBL entry number ENST00000376755 (SEQ ID NO:3), was isolated by a SuperscriptTM One-Step RT-PCR amplification kit with Platinum® Taq (Invitrogen) and a human total RNA pool panel as template (Human Total RNA, BD Biosciences Clontech). Flanking restriction sites Notl and Ascl were introduced into the fragment through the PCR amplification primers, to allow in-frame cl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com