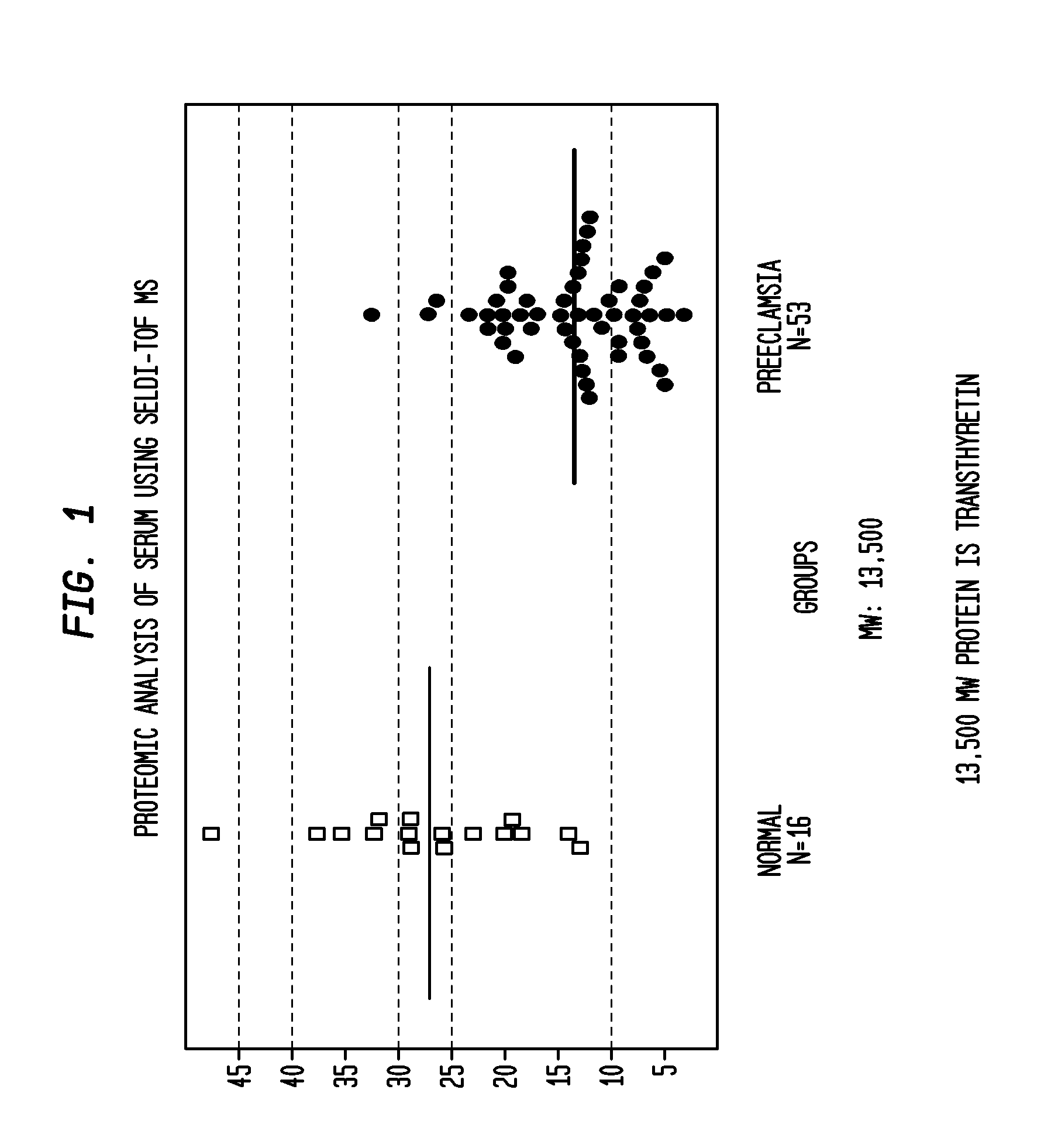
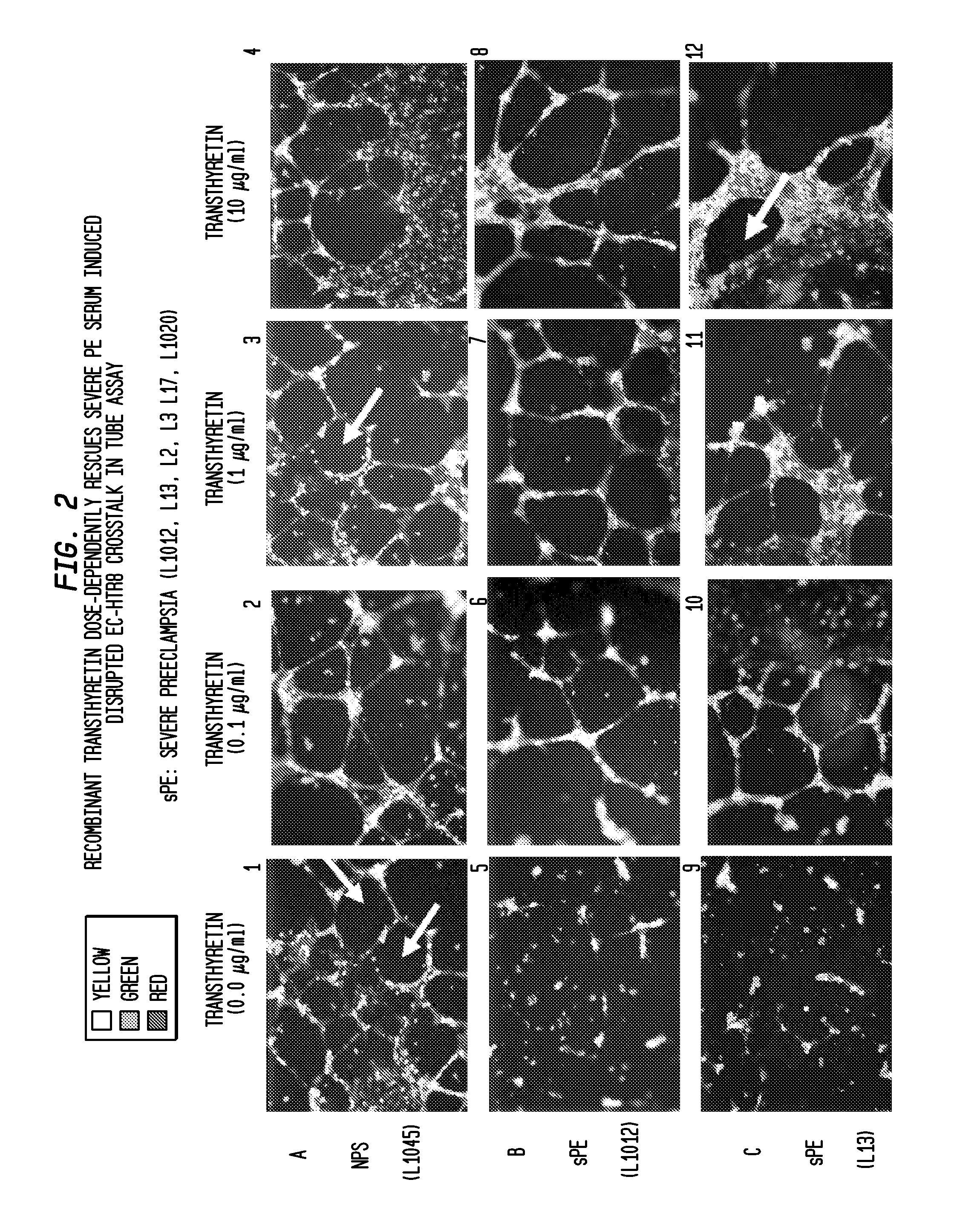
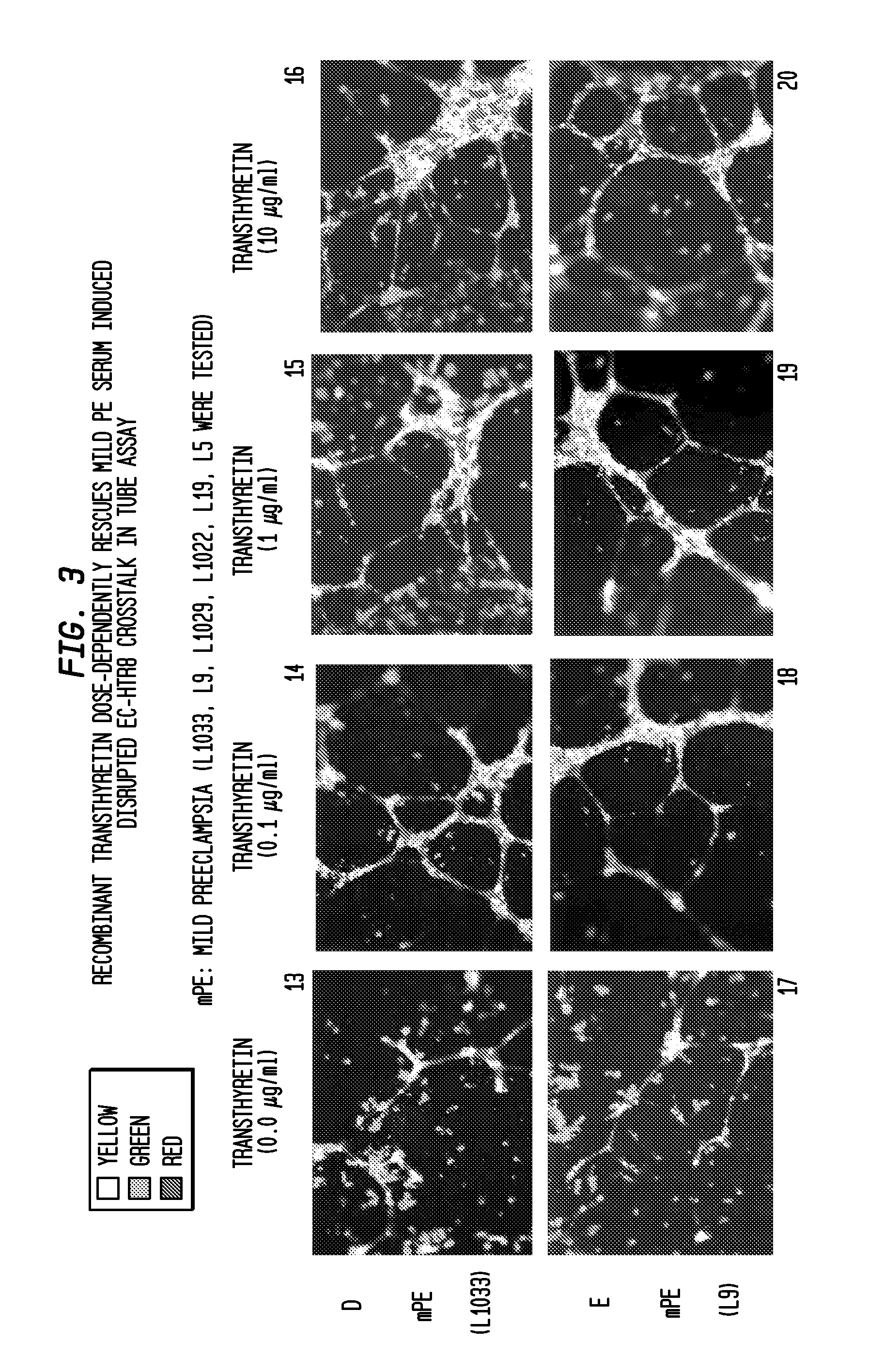
Compositions, formulations and methods of treating preeclampsia-type disorders of pregnancy
a technology of preeclampsia and formulation, applied in the direction of drug compositions, peptide/protein ingredients, peptide sources, etc., can solve the problems of poor spiral artery remodeling and placental ischemia, shallow placentation, maternal and infant illness and death,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CLINICAL EXAMPLE 1
Severe Preeclampsia
[0053]A pregnant woman at week 24 of gestation presents herself for routine checkup. Blood pressure measurements show systolic blood pressure is >150 mmHg (hypertension) and urine analysis show excess excretion of protein and creatinine (proteinuria, >1.5). The patient is diagnosed as severe preeclampsia. The subject is treated with a composition of recombinant or isolated human transthyretin protein at a dose of 100 mg / kg body weight over a 24 hour period and the levels of serum transthyretin are monitored by suitable detection method by ELISA or by SELDI-TOF. It is noted that in particular embodiments a dosage of 50-100 mg / kg of recombinant or isolated human transthyretin protein are therapeutic.
example 2
CLINICAL EXAMPLE 2
Mild Preeclampsia
[0054]A pregnant woman at week 28 of gestation presents herself for routine checkup. Blood pressure measurements show systolic blood pressure is >140 mmHg (hypertension) and urine analysis by ELISA show high protein to creatinine ratio (proteinuria) is >0.3, the patient is diagnosed as mild preeclampsia. The subject is treated with a composition of transthyretin protein at a dose of 40 mg / kg body weight over a 24 hour period and the levels of serum transthyretin are monitored by suitable detection method by ELISA or by SELDI-TOF. The clinical outcome of hypertension and proteinuria are monitored during the following week. Depending on the clinical diagnosis, a second dose of 40 mg / kg is given over a 24 hr period. It is noted that in particular embodiments a dosage of 25-50 mg / kg of recombinant or isolated human transthyretin protein are therapeutic.
example 3
CLINICAL EXAMPLE 3
Severe Preeclampsia
[0055]A pregnant woman at week 22 of gestation presents herself for routine checkup. Blood pressure measurements show systolic blood pressure is >155 mmHg (hypertension) and urine analysis show excess excretion of protein and creatinine (proteinuria, >1.5), the patient is diagnosed as severe preeclampsia. The subject is treated with a composition of transthyretin protein in combination with diclofenac 10:1 (mole / mole) at a dose of 10 mg / kg per day administered over a 24 hour period and the levels of serum transthyretin is monitored by suitable detection method by ELISA or by SELDI-TOF. The clinical outcome of hypertension and proteinuria are monitored in the following week. Depending on the outcomes, a second dose of 5-10 mg / kg of transthyretin-diclofenac is administered over a 24 hr period.
[0056]It is noted that in particular embodiments the mole ratio of transthyretin protein to diclofenac is from about 1:1 to about 10:1. It is further noted th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com