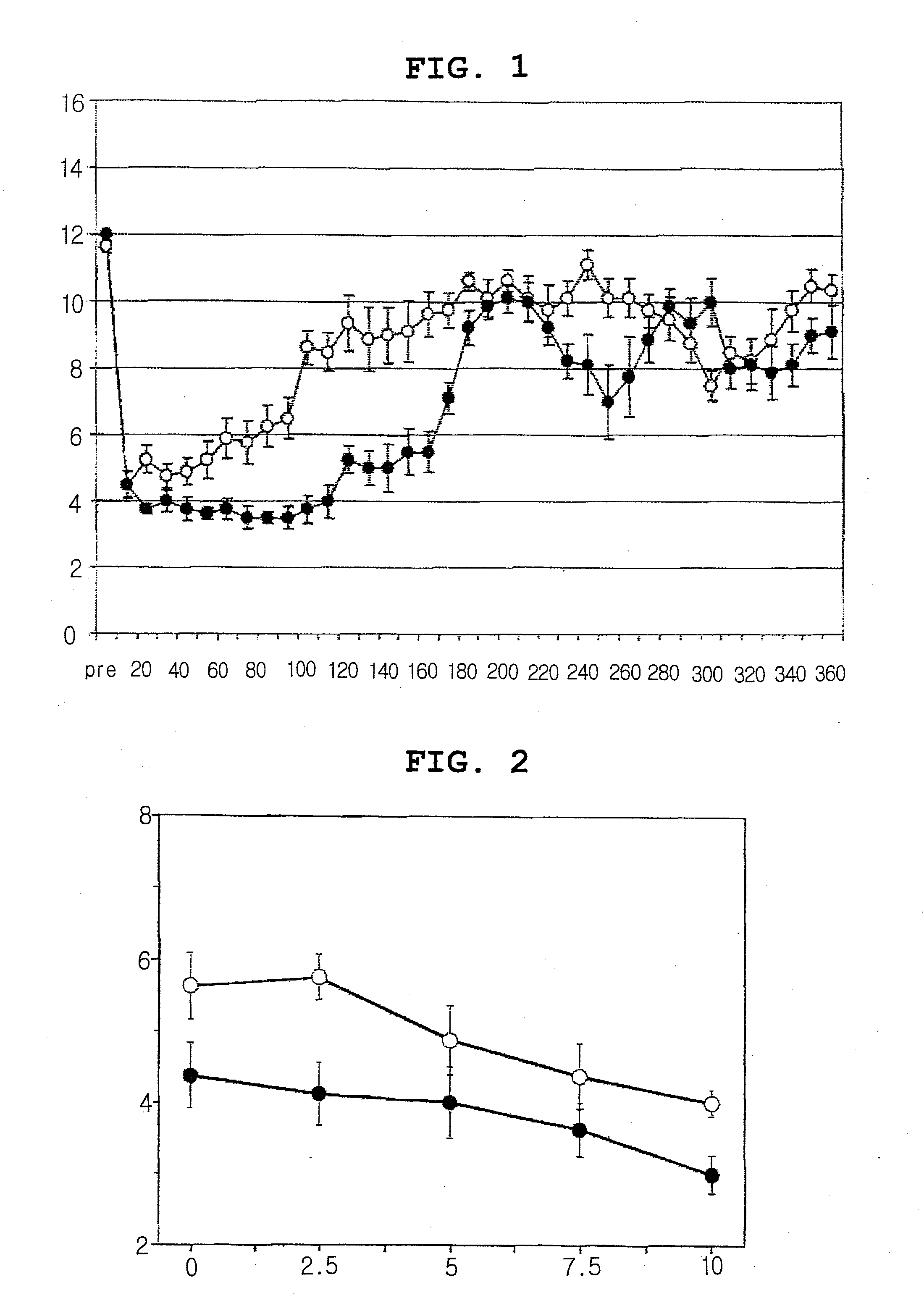
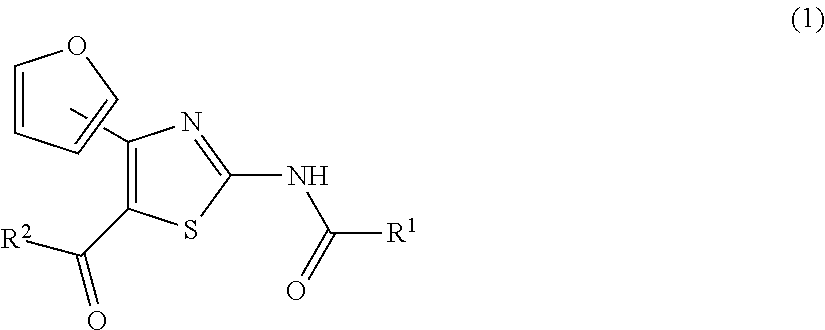
Therapeutic agent for motor disorders
a technology for motor disorders and therapeutic agents, applied in the direction of biocide, muscular disorders, drug compositions, etc., can solve the problems of motor fluctuations and dyskinesias, poor facial expression of parkinson patients, and increased disability with disease progression, so as to achieve treatment and/or prophylaxis of parkinson, and the effect of a movement disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0117]Tablets having the following formulations are prepared according to the conventional manner. Compound (IA) (40 g), lactose (286.8 g), and potato starch (60 g) are mixed, and then a 10% aqueous solution of hydroxypropylcellulose (120 g) is added thereto. The resulting mixture is kneaded according to the conventional manner, granulated, and dried to form granules for tableting. After adding thereto 1.2 g of magnesium stearate followed by mixing, the mixture is punched with a tableting machine having a punch measuring 8 mm in diameter (Model RT-15; Kikusui) to obtain tablets (containing 20 mg of an active ingredient per tablet).
TABLE 5FormulationCompound (IA)20mglactose143.4mgpotato starch30mghydroxypropylcellulose6mgmagnesium stearate0.6mg200mg
example 2
[0118]Tablets having the following formulation are prepared in the same manner as in Example 1.
TABLE 6FormulationCompound (IB)20mglactose143.4mgpotato starch30mghydroxypropylcellulose6mgmagnesium stearate0.6mg200mg
example 3
[0119]Tablets having the following formulation are prepared in the same manner as in Example 1.
TABLE 7FormulationCompound (IC)20mglactose143.4mgpotato starch30mghydroxypropylcellulose6mgmagnesium stearate0.6mg200mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com