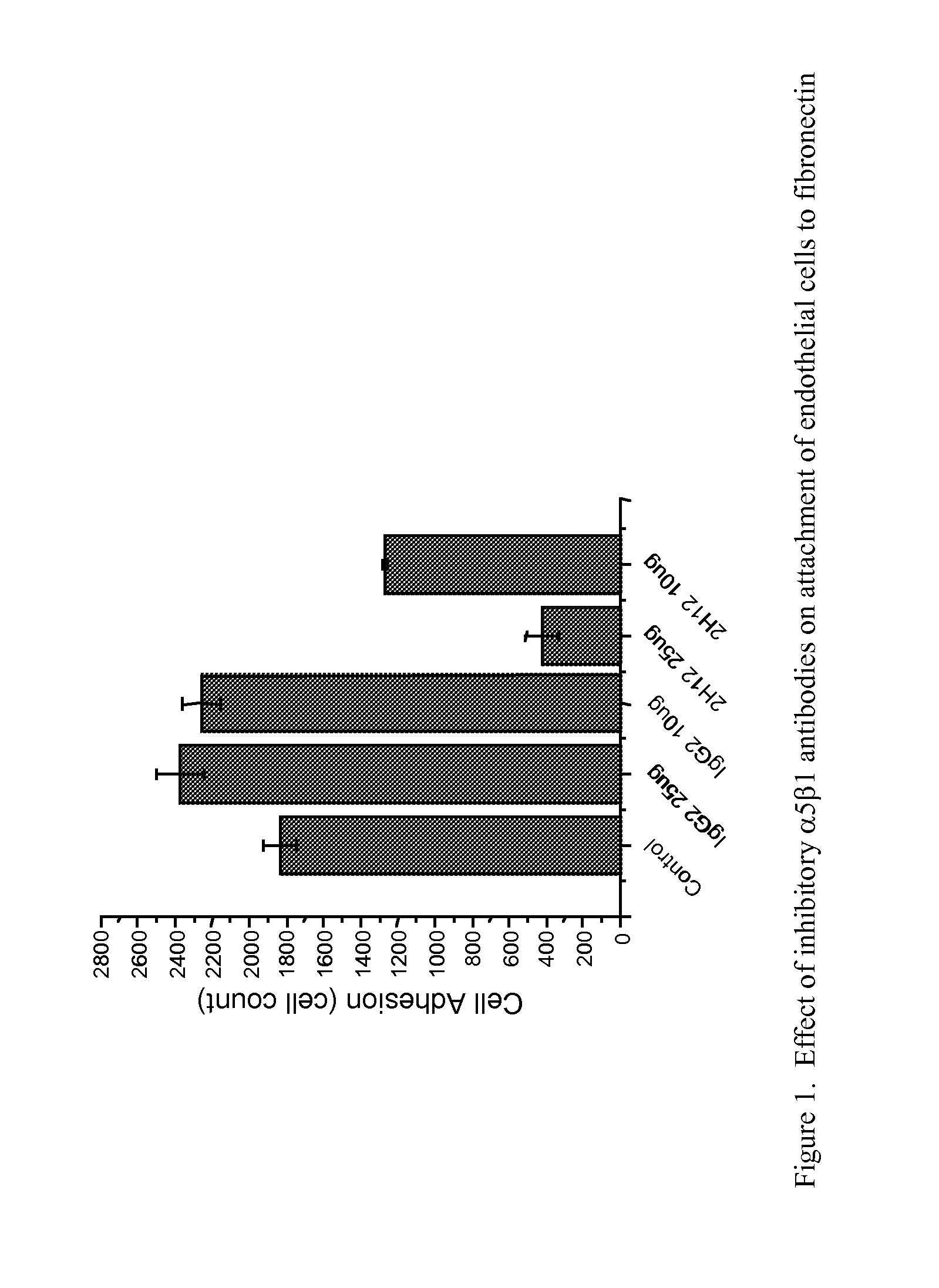
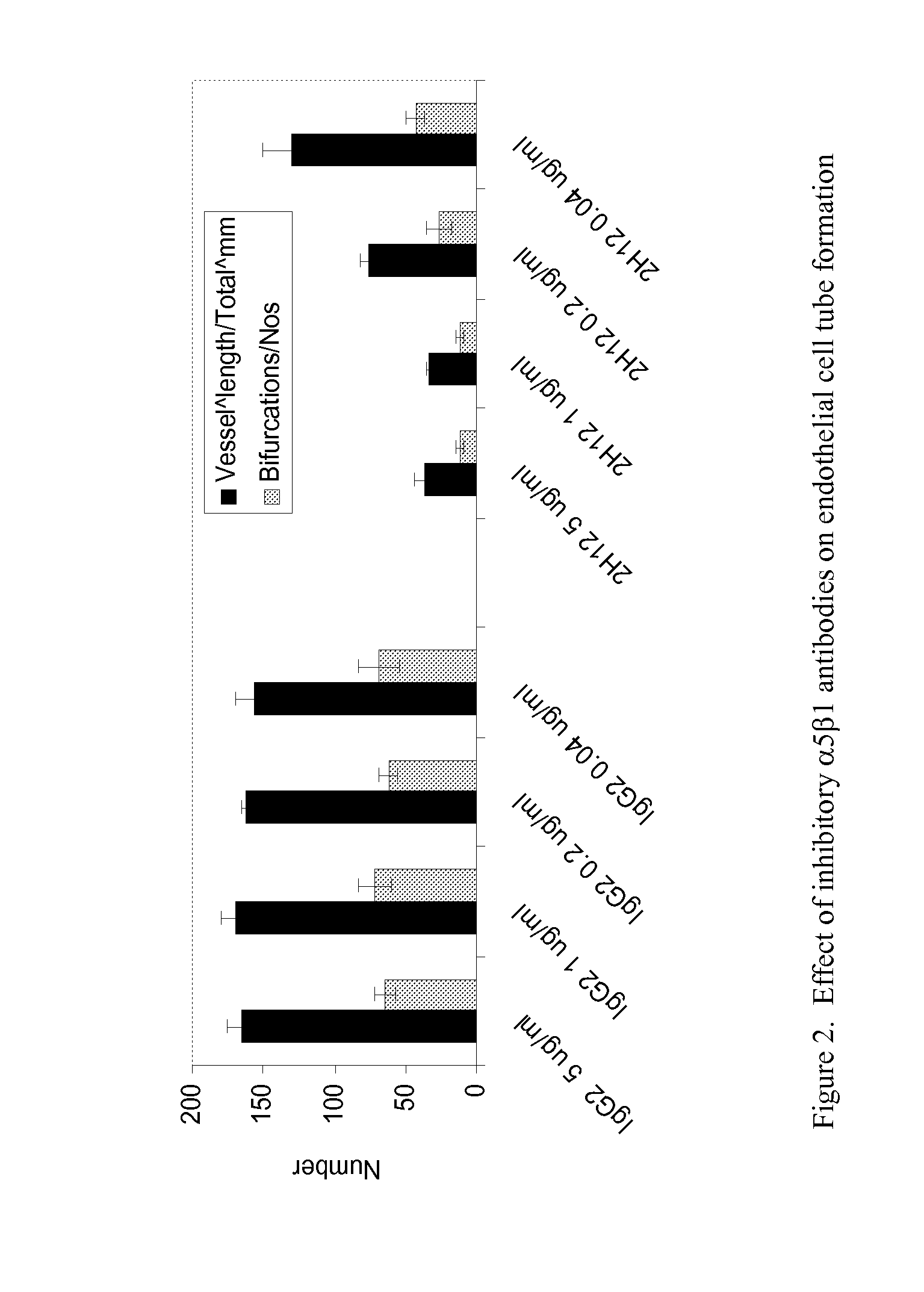
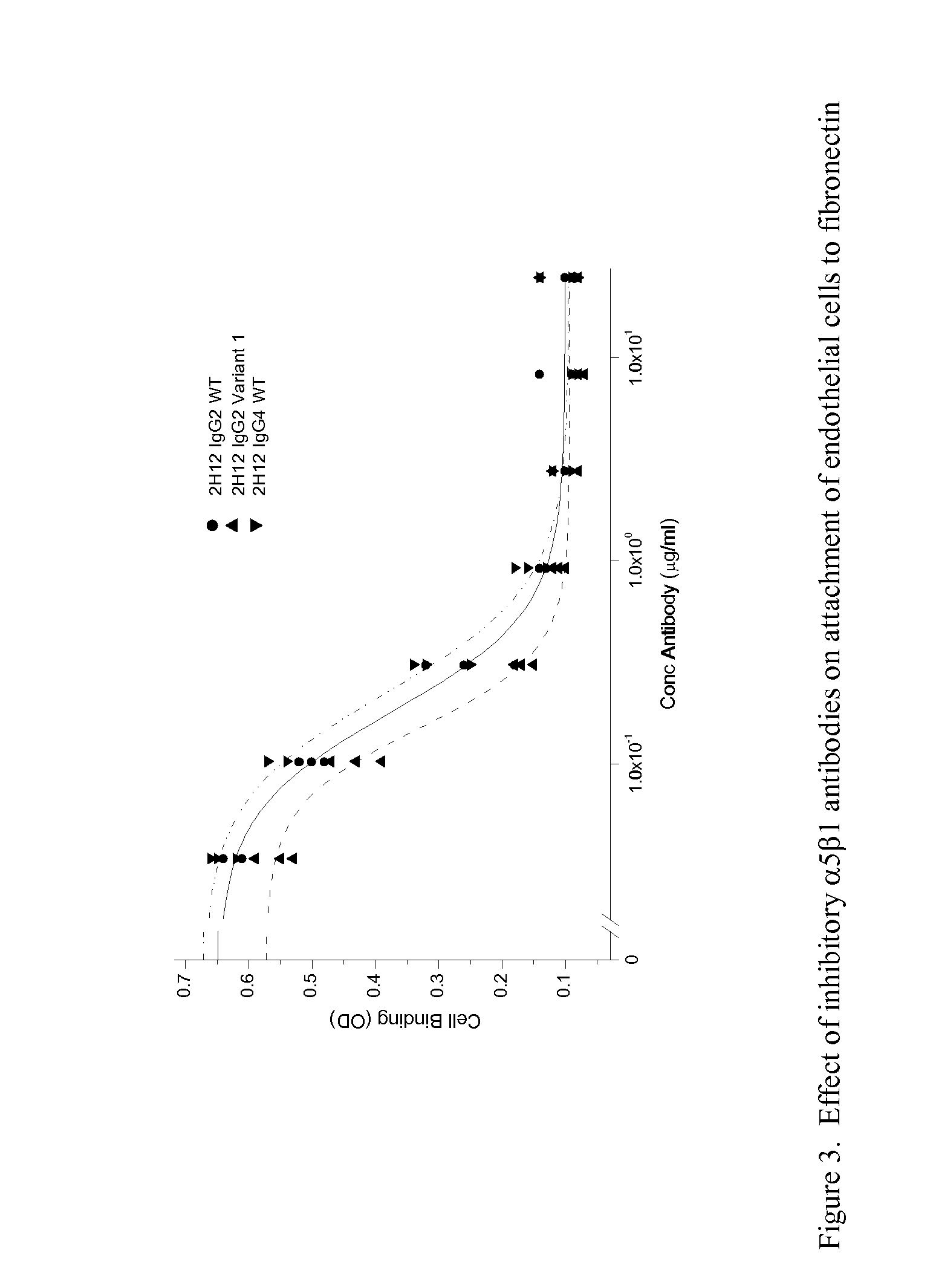
TARGETED BINDING AGENTS DIRECTED TO a5 1 AND USES THEREOF
a technology of target cells and binding agents, applied in the field of target cells, can solve problems such as similar embryonic lethality, and achieve the effects of enhancing the ability of fixing complement, and enhancing the ability of activating effector cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunization and Titering
Immunization
[0302]Immunizations were conducted using soluble α5β1 and cell-bound α5β1 (CHO transfectants expressing human α5β1 at the cell surface), respectively. For the generation of CHO transfectants, human full-length α5β1 cDNA was inserted into the pcDNA 3 expression vector. CHO cells were transiently transfected via electroporation. Expression of human α5β1 on the cell surface at the level suitable for immunogen purpose was confirmed by Fluorescence-Activated Cell Sorter (FACS) analysis. For the campaign, an initial injection of 2×106 cells / mouse of transfected CHO cells (group 1 and 2) and 10 μg / mouse of soluble protein (Groups 3 and 4) were used for immunization in XenoMouse™. The immunization was carried out according to the methods disclosed in U.S. patent application Ser. No. 08 / 759,620, filed Dec. 3, 1996 and International Patent Application Nos. WO 98 / 24893, published Jun. 11, 1998 and WO 00 / 76310, published Dec. 21, 2000, the disclosures of whi...
example 2
Recovery of Lymphocytes, B-Cell Isolations, Fusions and Generation of Hybridomas
[0304]Immunized mice were sacrificed by cervical dislocation, and the draining lymph nodes harvested and pooled from each cohort. Three independent harvests were conducted. Harvest 1 used five mice from group 1 with ID numbers 150927, 150928, 150929, 150930, 150031. Harvest 2 used six mice from group 2 with ID numbers 151037, 151038, 151039, 151040, 150588, 150589. The third harvest used five mice from group 1 with ID numbers 150932, 150919, 150920, 150921, 150926.
[0305]The lymphoid cells were dissociated by grinding in DMEM to release the cells from the tissues and the cells were suspended in DMEM. The cells were counted, and 0.9 ml DMEM per 100 million lymphocytes added to the cell pellet to resuspend the cells gently but completely. Using 100 μl of CD90+ magnetic beads per 100 million cells, the cells were labeled by incubating the cells with the magnetic beads at 4° C. for 15 minutes. The magneticall...
example 3
Antibody Titer Measurement: Native Antigen Binding of 300.19 Cells
[0310]FACS analysis was performed on 300.19 cells to measure the titers of antibody against α5β1 expressed on B300.19 cells. 300.19 cells (control and transfected with human α5β1) were seeded at 50,000 cells / well and incubated with 90 μL of sample supernatant (at 1:50 dilution) for one hour at 4° C. The wells were then washed and incubated with Cy5-conjugated goat anti-human antibody (Jackson Laboratories) at 5n / mL and 7-Amino-Actinomycin (7AAD) at 5 μg / mL for 15 minutes at 4° C. Bound α5β1 was detected using FACS analysis. The positive control was mouse anti-α5β1 antibody (R&D Systems, Inc.), and negative controls included goat anti-human and anti-mouse Fc Cy5 coupled antibody (Jackson Laboratories) alone, as well binding or irrelevant mouse IgG1 and human IgG2, irrelevant supernatants as indicated. Animals with the greatest FACS Geometric Mean Fluorescence were selected for subsequent hybridoma generation. Table 3 l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com