Advanced analyte sensor calibration and error detection
a technology of analyte sensor and calibration method, which is applied in the field of advanced analyte sensor calibration and error detection, can solve the problems of increased glucose concentration, dangerous side effects, and diabetics may not know whether their blood glucose value is increasing or decreasing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sensitivity and Impedance Relationship
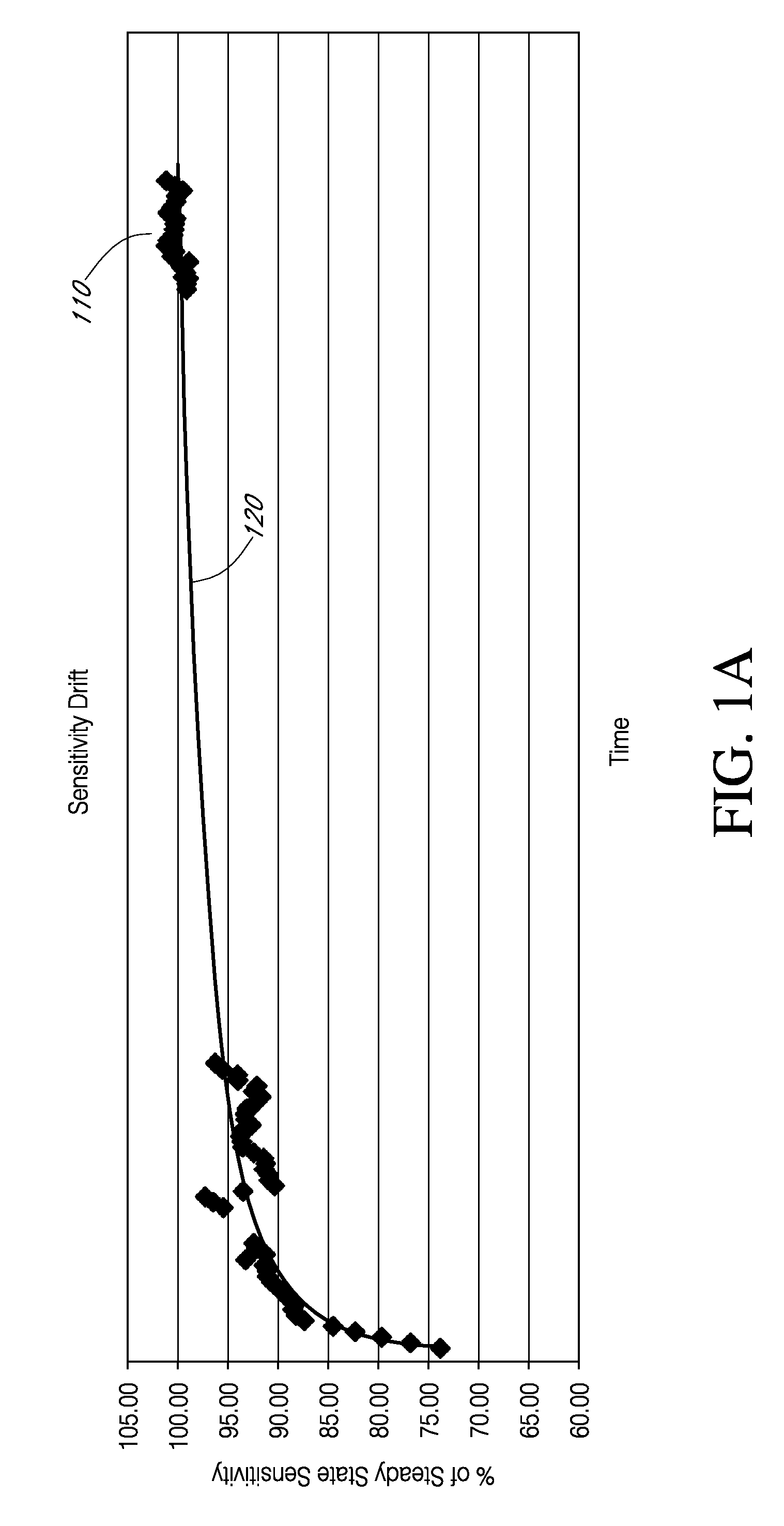
[0433]Example 1 illustrates a relationship between sensitivity of a sensor and an impedance of the sensor. In this example, an IVBG sensor was connected to a Gamry potentiostat system and placed in a in a buffer solution of a modified isolyte having a glucose concentration of 100 mg / dL. The temperature during the experiment was 37 C. An impedance spectrum was captured at fixed intervals of time. The impedance spectrum analyzed in this experiment ranged from 1 Hz to 100 kHz and measurements of the sensor impedance and sensor sensitivity were taken at 15 minute intervals over a period of about 1200 minutes.
[0434]Reference is now made to FIG. 25, which is a graph showing absolute values of sensitivity and impedance of the sensor based on an input signal having a frequency of 1 kHz. Data points 2502 represent measured values of sensor sensitivity over a time period of 1200 minutes (20 hours), where t=0 corresponds to the time when the sensor is init...
example 2
Retrospectively Compensating for Sensitivity Drift Using Impedance
[0437]FIG. 27 is a plot of sensitivity and impedance points measured at various intervals over time using seven different sensors, Sensors A-G. Sensors A-G were transcutaneous-type sensors, but selected from several different sensor lots. Thus, even though Sensors A-G were all transcutaneous sensors, sensors from different lots may have been made in a slightly different way or under slightly different conditions, which can result in sensors from different lots exhibiting different sensitivity profiles. In this Example, Sensors A and D were selected from a first lot, Sensor B was selected from a second lot, and Sensors C, E, F and G were selected from a third lot.
[0438]Further to FIG. 27, the plotted data points are sensitivity and impedance values for each Sensor A-G. Because the sensitivity of each Sensor A-G gradually increases over time, the right most points of each Sensor's plotted data points tend to correspond ...
example 3
Prospective Calibration of Sensor Data Using Impedance Measurements
[0447]Example 5 pertains to prospective calibration. Further, in this experiment, calibration of sensor data is based on a change of sensitivity to change in impedance relationship previously derived from sensors from a different sensor lot. That is, in Example 3, the estimative curve 2802 is used to compensate data obtained using Sensors R-U, each of which was selected from a fourth sensor lot, the fourth sensor lot was not included in the group of sensors used to derive the estimative curve 2802. Example 5 shows that data can be calibrated using a change in sensitivity to change in impedance relationship derived from different sensor types than the type of sensor being calibrated. This can indicate that a sensor factory calibration code need not be used to compensate for sensitivity drift.
[0448]FIGS. 35 and 36 illustrate prospectively calibrating sensor data obtained from Sensors R-U. FIG. 35 is a plot of the perce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com