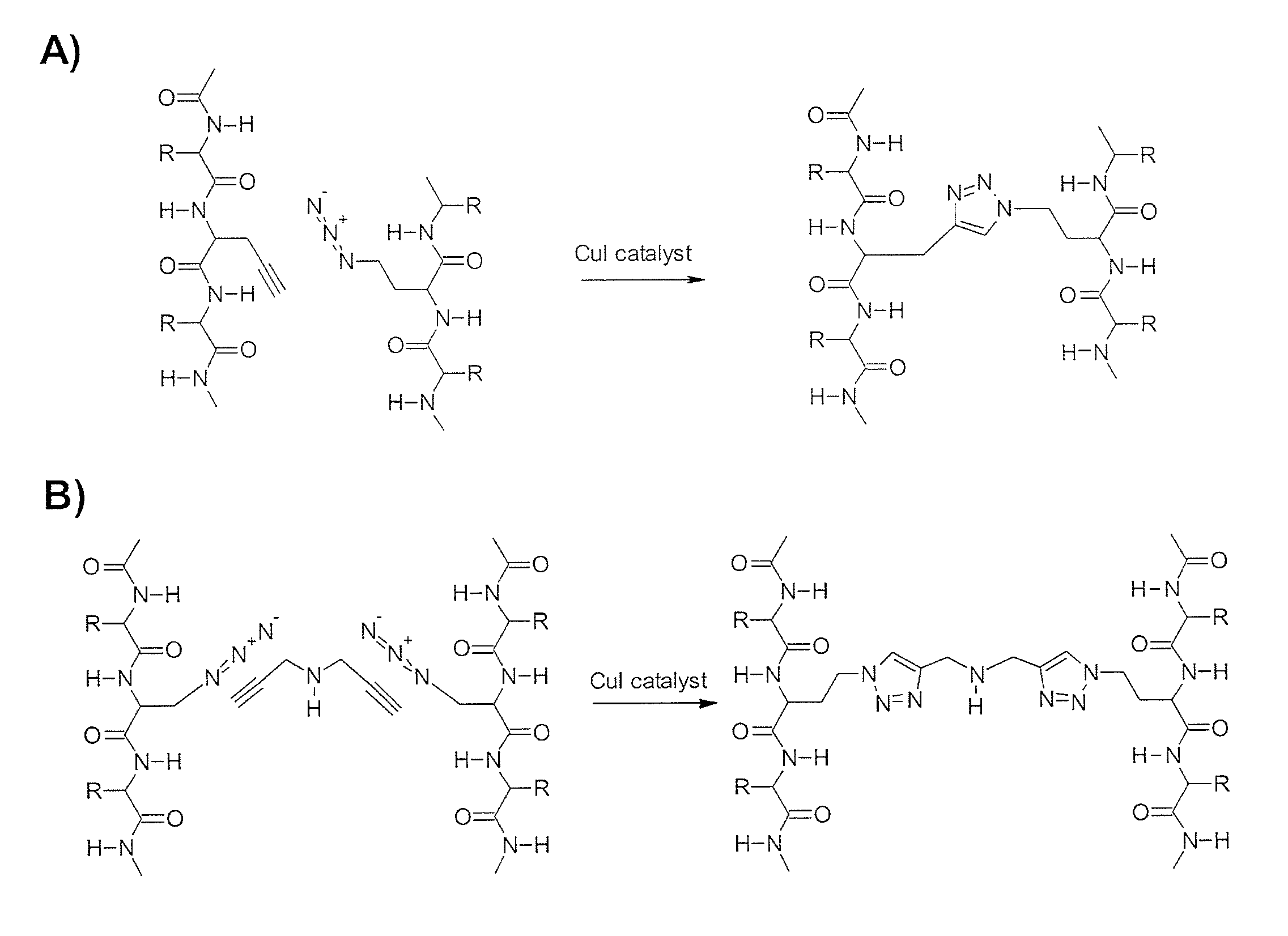Apoe peptide dimers and uses thereof
a technology of apoe peptide and dimers, which is applied in the direction of peptide sources, metabolic disorders, extracellular fluid disorders, etc., can solve the problems of limited efficacy, adverse side effects or limited effects, and the efficacy of surgical removal of tumors from cancerous tissue is often limited, so as to increase the potency and increase the biological activity of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Cytotoxic Activity of ApoE Peptides is Enhanced by Formation of Disulfide Dimers
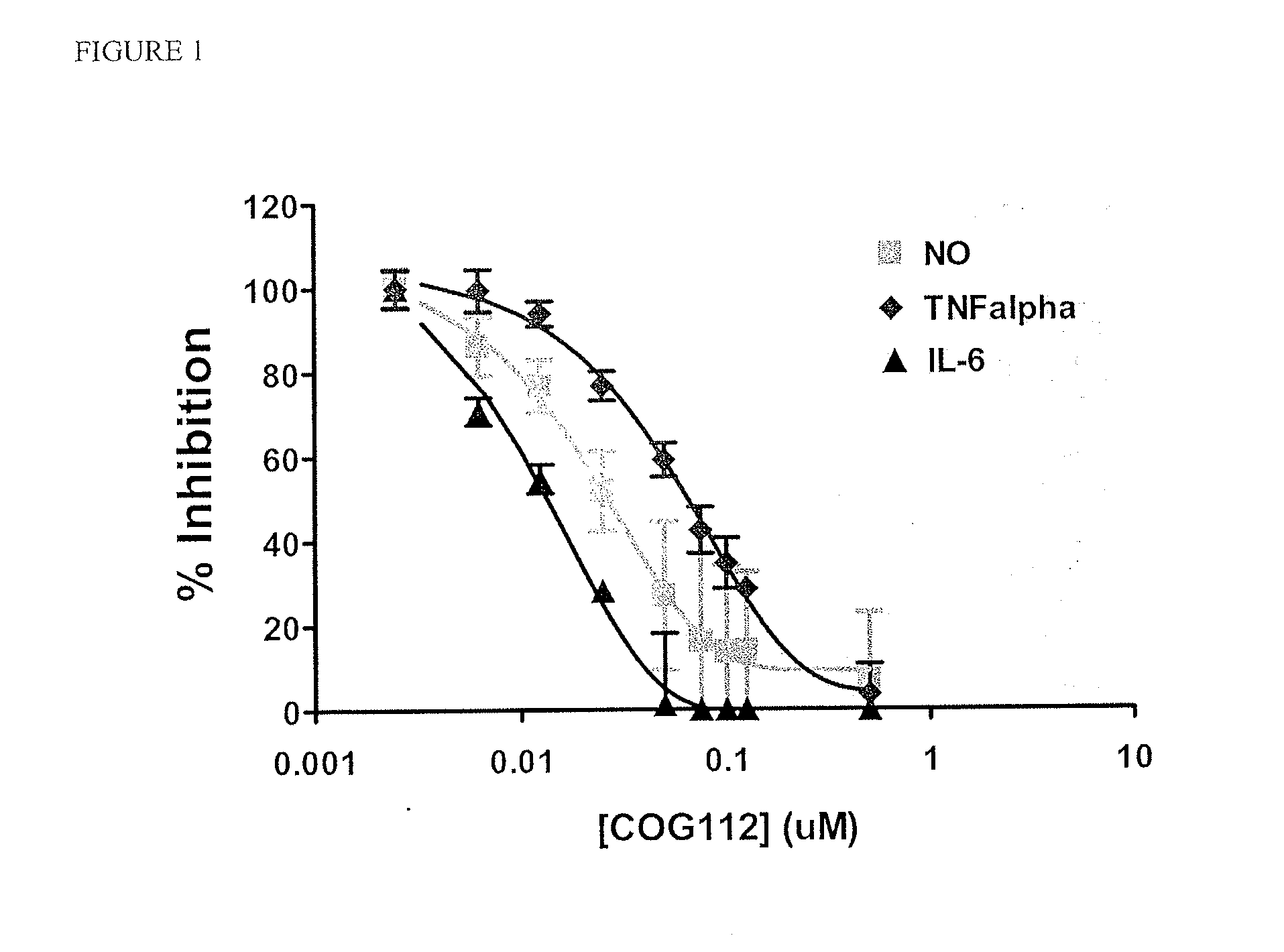
[0099]We have previously shown that the addition of a protein transduction domain (PTD), such as an antennapedia peptide, to the apoE-mimetic COG133 peptide (LRVRLASHLRKLRKRLL (SEQ ID NO: 3)) enhances its anti-inflammatory activity. A series of fusion peptides with COG133 conjugated to a PTD were prepared by chemical synthesis. Notably, we found that COG112 with the sequence RQIKIWFQNRRMKWKKCLRVRLASHLRKLRKRLL (SEQ ID NO: 1), was effective in suppressing production of NO, TNFα and IL-6 with IC50s of 21 nM, 58 nM, and 12 nM, respectively, in BV2 cells following stimulation with LPS (FIG. 1). These results demonstrate a significant safety window for COG112 where effective suppression occurs at concentrations of 12-58 nM while the LD50 is >120-fold higher at 7 μM.
[0100]During the course of testing various compounds for cytotoxicity against CLL cells, we found that COG112 had an ED50 of 220 nM. These data...
example 2
Non-Reducible COG112 Dimer Peptides Activate PP2A and are Cytotoxic to Cancer Cells
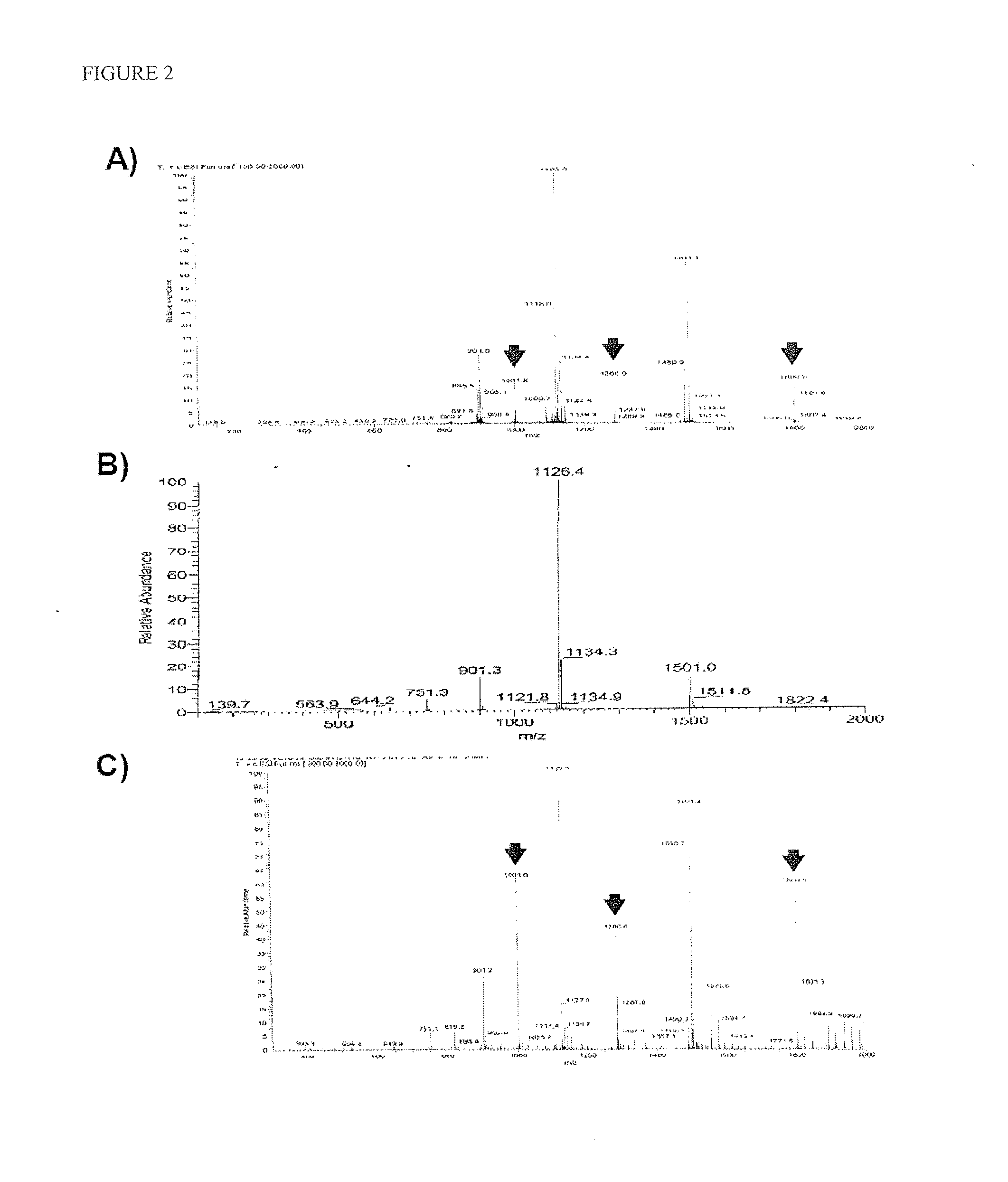
[0104]After discovery that COG112 was active as a disulfide-linked dimer, we sought a method to stabilize the dimer state of COG112. We initially treated the reduced COG112 peptide with a 5-fold molar excess of bismaleimidoethane (BMOE) in dilute solution. The peptide was precipitated by addition of ether, collected by filtration, and the unreacted BMOE removed by washing prior to drying under vacuum. The BMOE-linked peptide was dissolved in buffer and mixed with a 1.5-2.0 molar excess of freshly reduced COG112. Coupling was monitored by HPLC until the reaction was complete and the resultant peptide-BMOE-linker-peptide dimer was precipitated with ether, collected, washed, and purified by reverse phase HPLC to a purity of 98%. The identity of this peptide (known as COG449) was confirmed by MS and was assayed in the BV2 NO release assay. As shown in FIG. 3, we observed an IC50 of 9.4 nM for nitric oxide...
example 3
Creation of an ApoE Peptide Dimer Library
[0112]Based on the results demonstrated in Examples 1 and 2 that dimers of ApoE peptides are more potent in inducing cytotoxicity of cancer cells and activating PP2A, twenty eight different monomer ApoE peptides are synthesized that can be coupled with two different coupling chemistries to create a dimer library of sixty four unique compounds. The goal of this Example is to establish a library of chemically stable peptide dimers designed to explore the structure activity relationship between apoE-mimetic peptides and cytotoxicity for CLL cells. Our initial screens with COG peptides were limited to a single dimer peptide, COG112, which has the sequence Ac-RQIKIWFQNRRMKWKKCLRVRLASHLRKLRKRLL-amide (SEQ ID NO: 1) that contained a disulfide bridge through the cysteine at position 17. This peptide has an antennapedia-derived PTD domain at the N-terminal end and an apoE-mimetic domain in the C-terminal portion such that the dimerized peptide contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com