Method for assessment of potential for development of dravet syndrome and use thereof
a potential development and dravet syndrome technology, applied in the field of assessing a potential development potential of dravet syndrome, can solve the problems of easy induced convulsion seizure, hinder motivation, and worsen the effect of dravet syndrome and intractableness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Risk Factors for Predicting Development of Dravet Syndrome
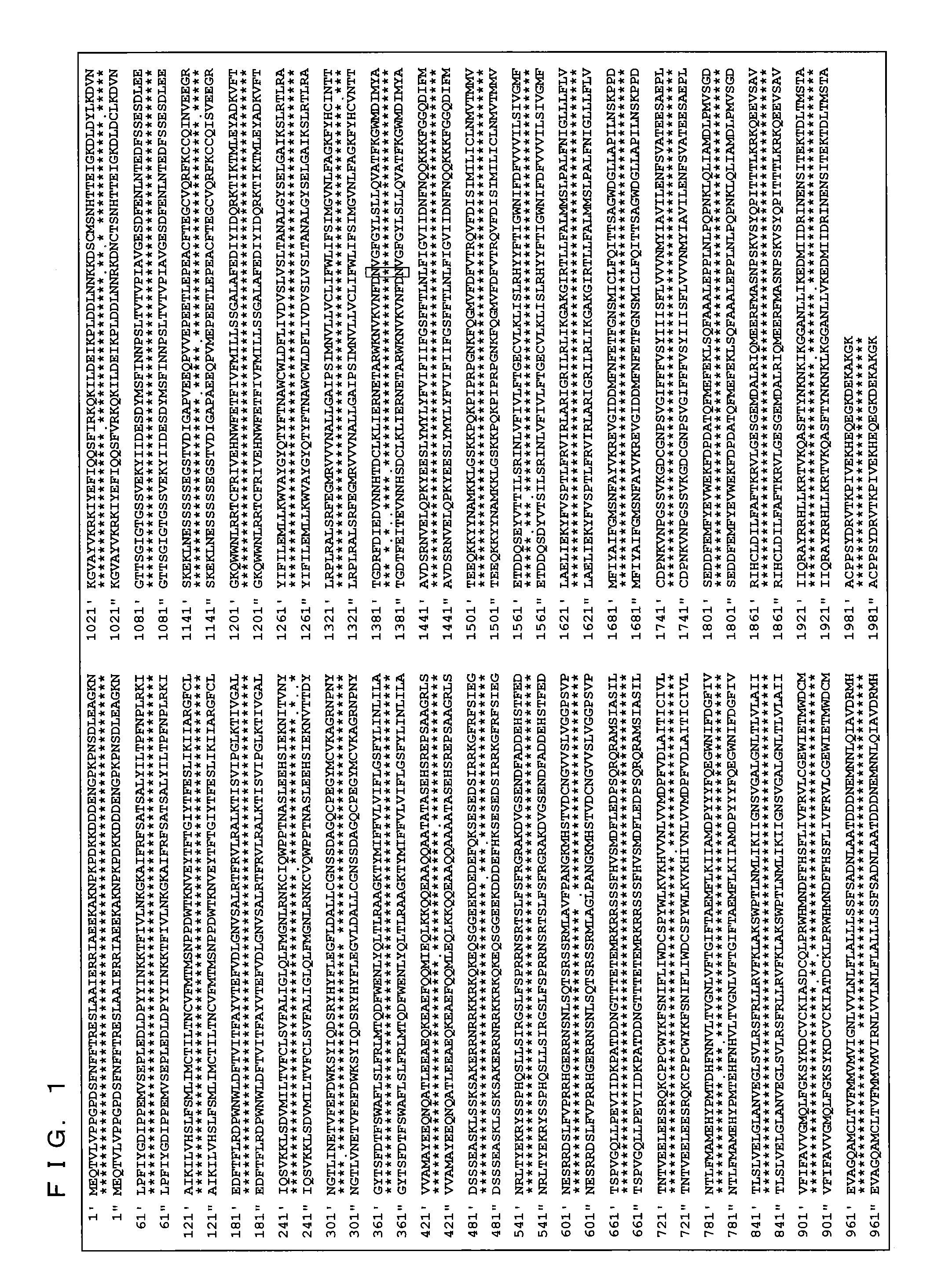
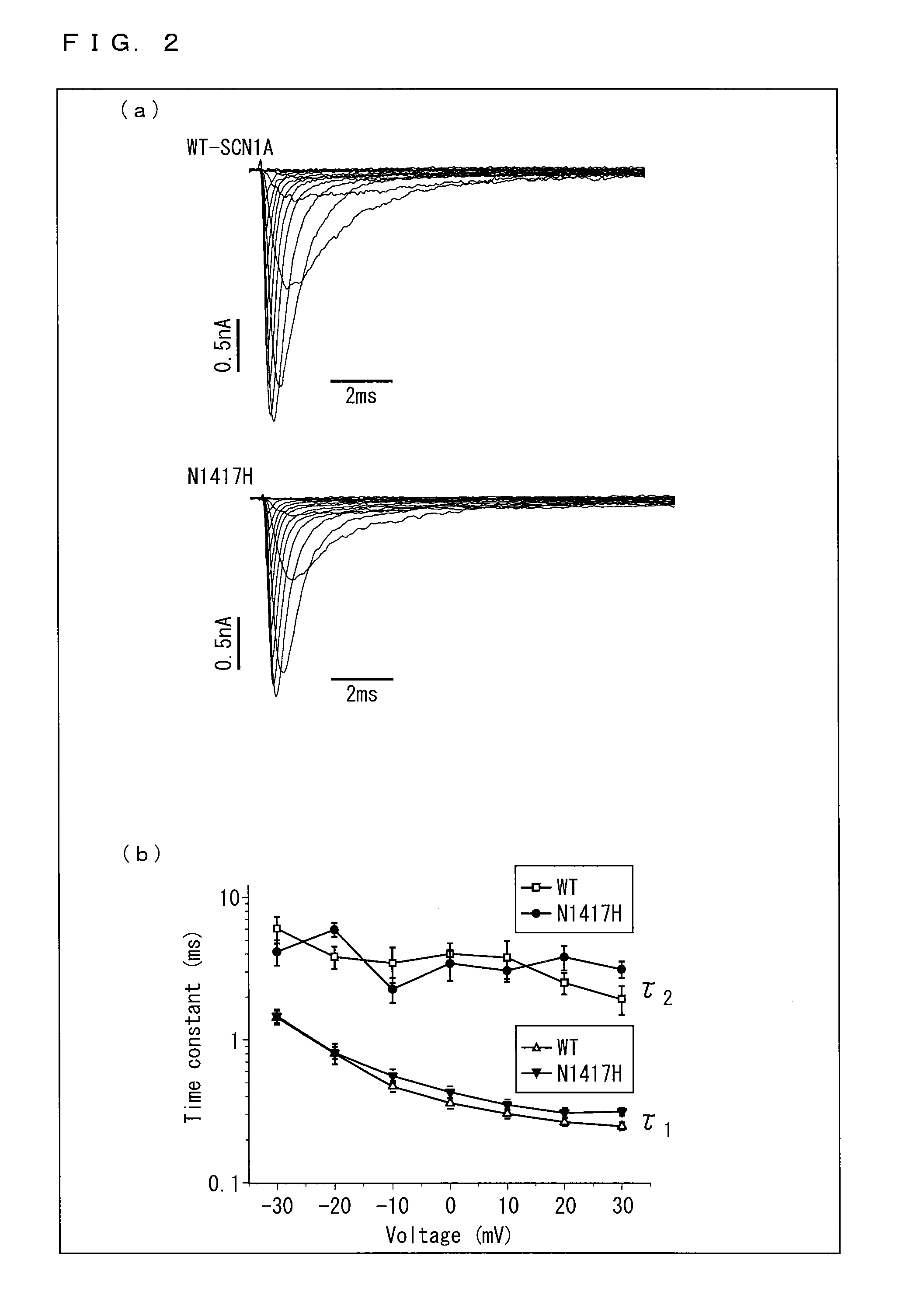
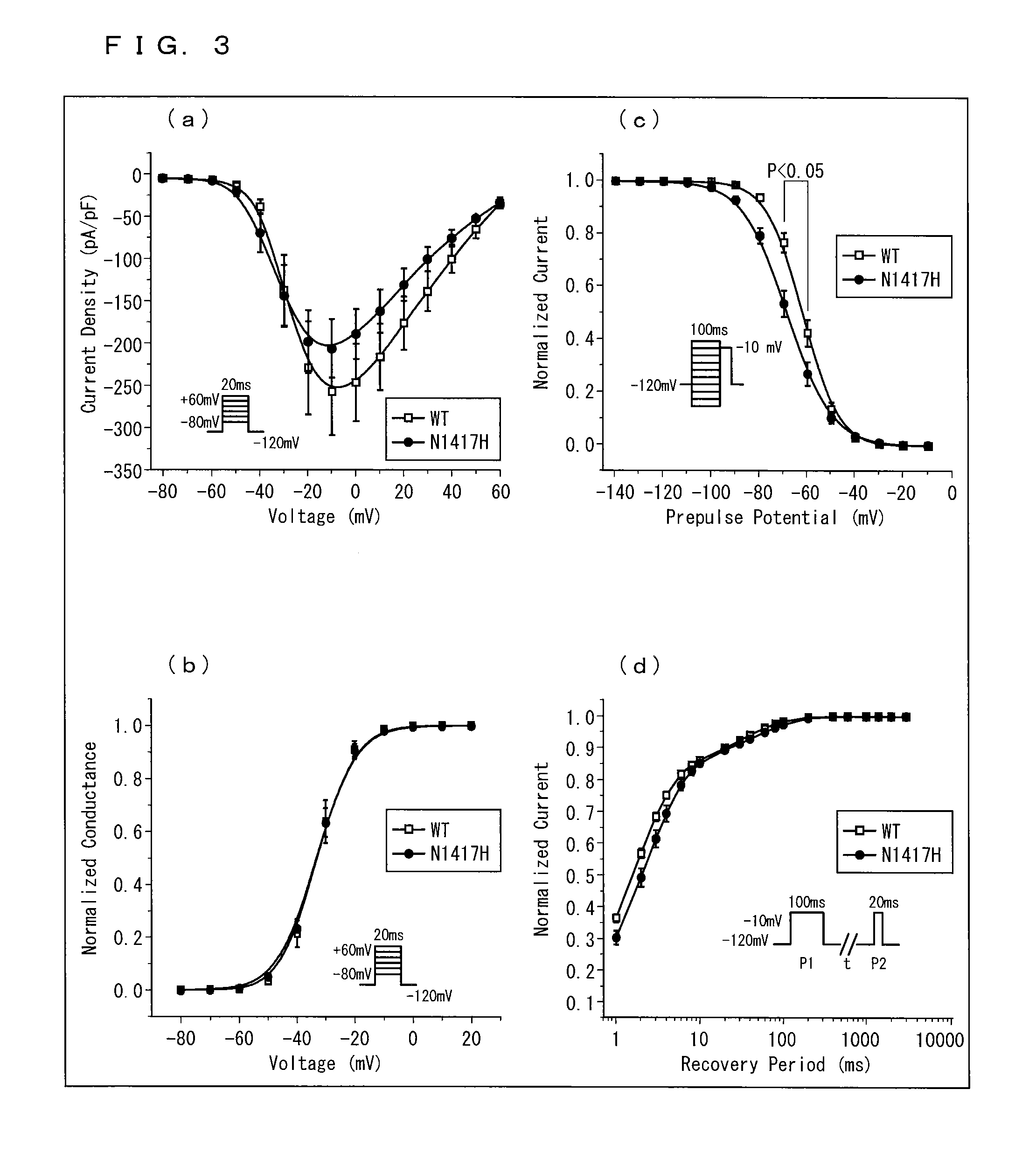
[0264]DNA were extracted from peripheral blood of 47 Dravet syndrome patients who visited Okayama University Hospital and / or its related hospitals, and mutations on various genes were analyzed. This study was performed upon receiving approval from Okayama University, Institutional Review Board of Human Genome and Gene Analysis Research.
[0265]More specifically, a genomic DNA was extracted from peripheral blood of a patient with use of a DNA extraction kit (WB kit; Nippon gene, Tokyo, Japan), and all exons were amplified by PCR. In PCR, a reaction solution of 25 μl was used, which includes 50 ng of human genomic DNA, 20 μmol of various primers, 0.8 mM of dNTPs, 1 reaction buffer, 1.5 mM of MgCl2, and 0.7 units of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, Calif., USA). As to the nucleotide sequence (SEQ ID NOs.: 9-62) of the primer pair used, see “Sequence of primers” described later.
[0266]...
example 2
Study of Gene Mutation in Benign Febrile Seizure Patient
[0295]A study was performed of a SCN1A gene and CACNA1A gene abnormality in a benign febrile seizure patient. DNA was extracted from peripheral blood of 50 patients of benign generalized epilepsy with febrile seizure plus (GEFS+), who visited Okayama University Hospital and / or its related hospitals, and mutations on various genes were analyzed. The DNA extraction, PCR amplification of the gene, and sequencing reactions were performed by the methods described above.
[0296]First, mutation analysis of voltage-gated sodium ion channel SCN1A gene was performed, which resulted in detecting gene mutation that caused amino acid changes in 6 patients. Next, mutation analysis was performed for 9 kinds of mutations of missense mutations and deletion mutations that were detected in the coding region of the CACNA1A gene, which resulted in detecting a mutation in 16 patients. Each of the mutations are shown in Table 5.
TABLE 5SCN1A and CACNA1A...
example 3
Study of Gene Mutation in a Healthy Person
[0302]To investigate whether the remaining 6 kinds of gene mutations excluding the registered 3 kinds out of the 9 kinds of missense mutations and deletion mutations detected in the coding region of the CACNA1A gene are of the gene polymorphism (SNP), gene mutation of the CACNA1A gene was similarly analyzed for DNA extracted from blood of 190 healthy persons. Results of the 9 kinds of the missense mutations and deletion mutations detected in the coding region of the CACNA1A gene are shown in Table 6. As a result, one kind of the CACNA1A gene mutation (G266S) was not detected from the healthy persons. From this result, it was found that the CACNA1A gene mutation of G266S is not an SNP, and is a novel gene mutation (gene abnormality) not found in the 190 healthy persons, which neither is in the NCBI SNP database.
TABLE 6CACNA1A gene mutation detected in healthy persons and Dravet syndromeNucleotideAmino AcidControlExonSubstitutionSubstitutionDr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com