Compositions and Methods for Treating Glioblastoma GBM
a glioblastoma and composition technology, applied in the field of compositions and methods for treating glioblastoma multiforme, can solve the problems of increased aggressiveness, poor prognosis, increased malignancy and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
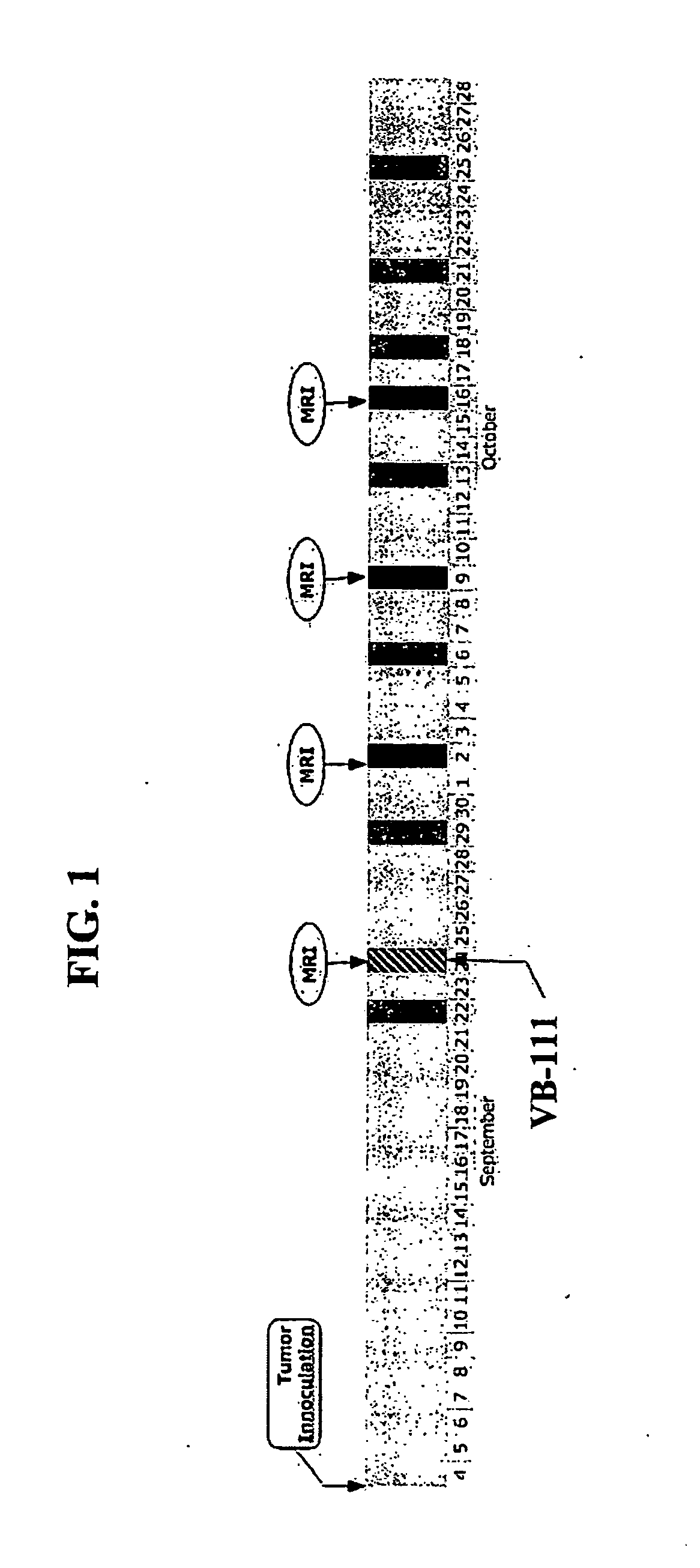
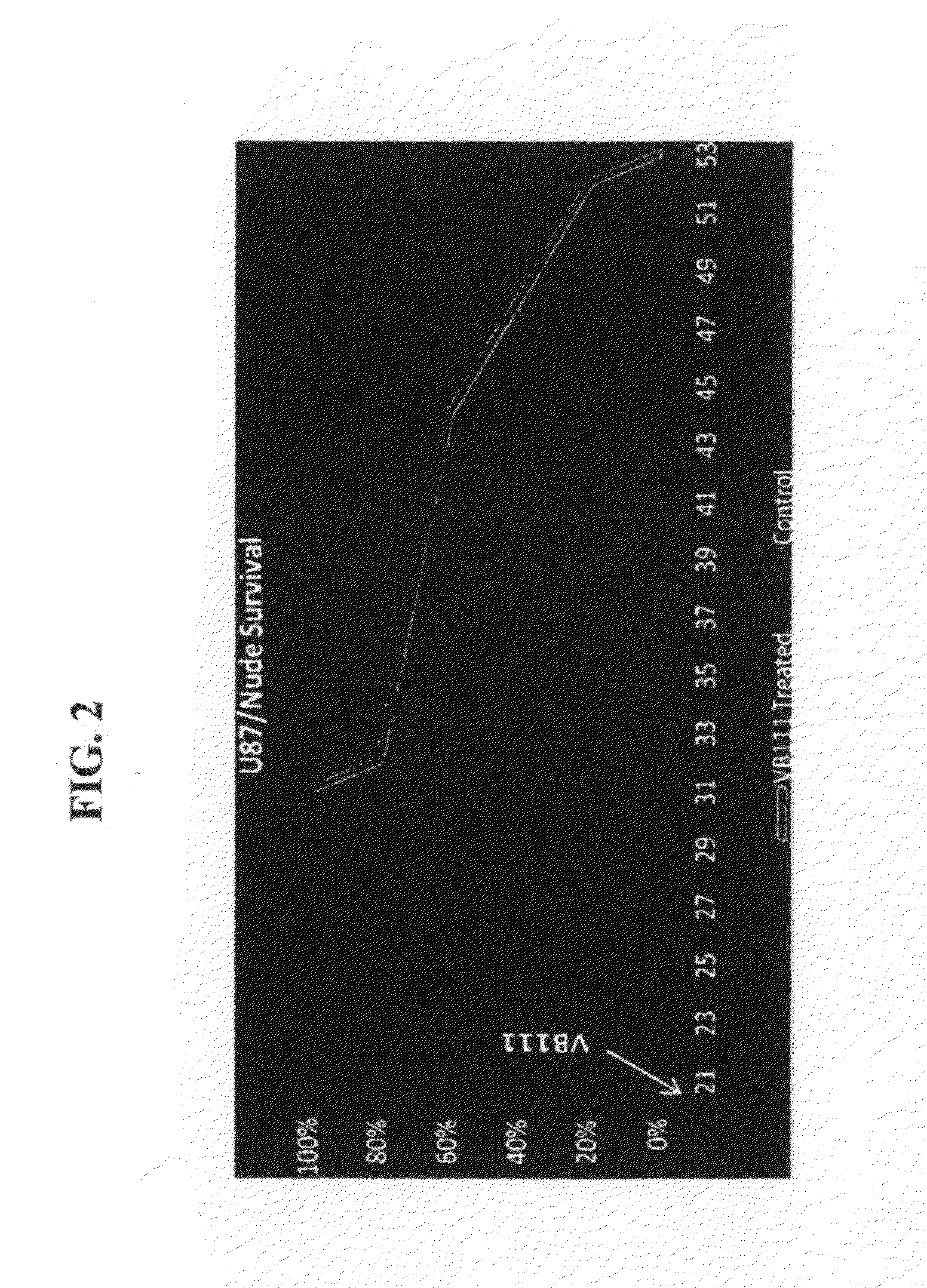
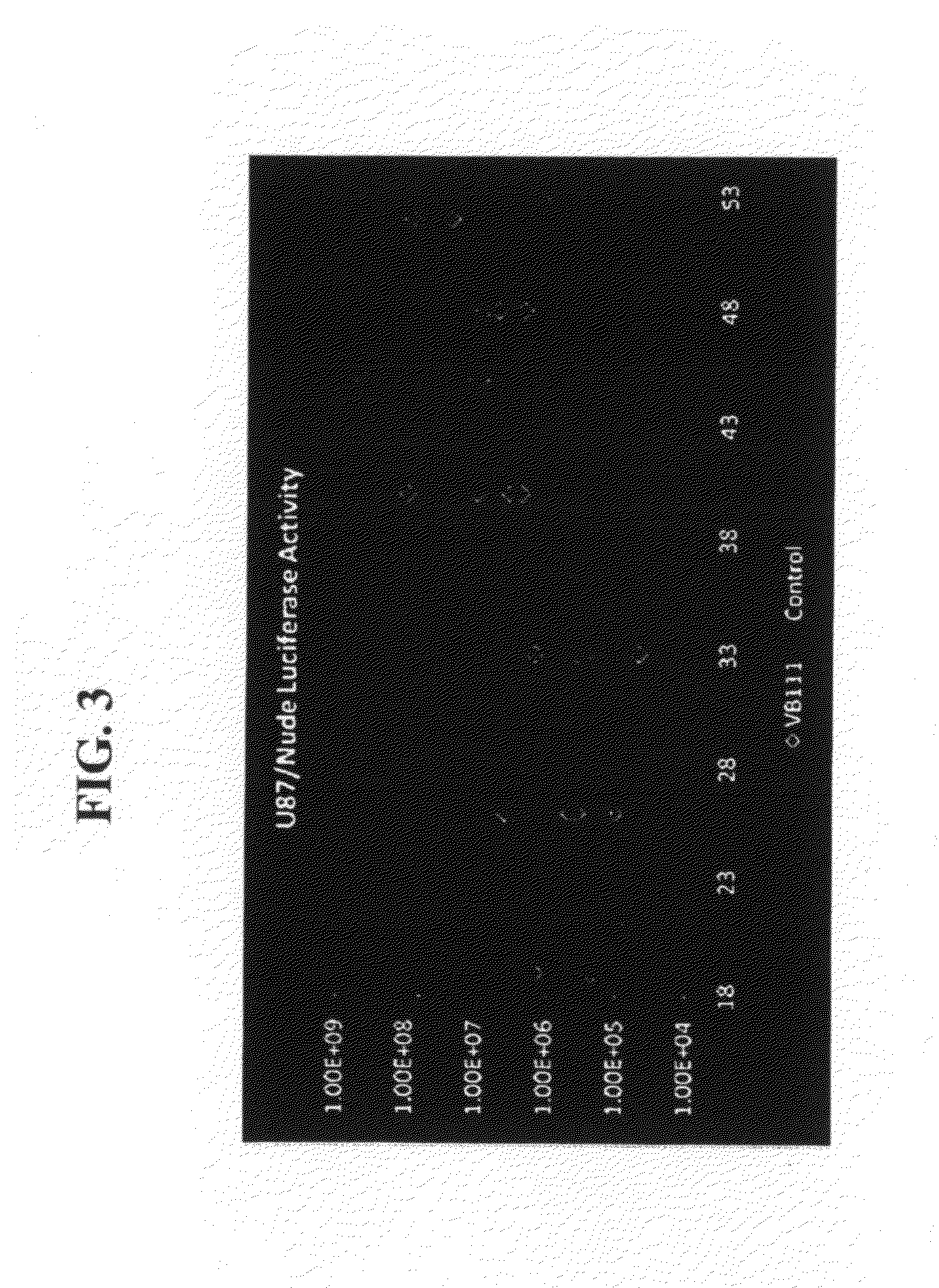
Effect of VB-111 in an Animal Model of Glioblastoma
[0336]Materials and Methods
[0337]Construction and Cloning of the Viral Vector:
[0338]The vector was constructed using a backbone containing most of the genome of adenovirus type 5, as well as partial homology to an adaptor plasmid, which enables recombination.
[0339]The E1 early transcriptional unit was deleted from the backbone plasmid, and further modified by deleting the pWE25 and the Amp resistance selection marker site.
[0340]The adaptor plasmid, containing sequences of the Ad5, CMV promoter, MCS, and SV40 polyA was modified to delete deleting the CMV promoter, and the PPE-1 promoter and Fas-c fragment were inserted by restriction digestion.
[0341]The modified PPE-1 promoter (PPE-1-3X, SEQ ID NO: 12) and the Fas-chimera transgene (Fas-c, SEQ ID NO: 4) were utilized for construction of the adenoviral vector. The PPE-1-(3X)-Fas-c element (2115 bp) was constructed from the PPE-1-(3X)-luc element. This element contains the 1.4 kb of th...
example 2
Effect of VB-111 in Glioblastoma Patients
[0359]Treatment Plan:
[0360]VB-111 will be administered as a single intravenous infusion of 1×1012 or 3×1012 Dose.
[0361]Study consists of 2 cohorts.
[0362]Cohort 1a: 3-6 subjects, safety (1×1012 VPs);
[0363]Cohort 1b: 3-6 subjects, safety (3×1012 VPs);
[0364]Cohort 2: 23-26 subjects, efficacy & safety (3×1012 VPs)
[0365]Cohort 1a & Cohort 1b: Study subjects will be enrolled sequentially. The first subject of each cohort will be treated and observed for 14 days; if no dose-limiting toxicities (DLT) are observed, then another two subjects will be recruited to that cohort. All six subjects of cohort 1 need to be observed for a minimum of 14 days and show no DLT for the start of the next cohort. If a DLT is observed in one patient in a specific dosing cohort, three additional subjects will be accrued for the same dosing cohort, and safety will be reassessed. If DLT is confirmed, i.e. two out of six subjects experience a DLT, then the study will be dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com