Radioiodination method
- Summary
- Abstract
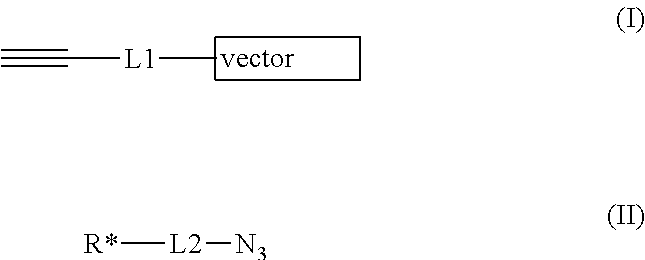
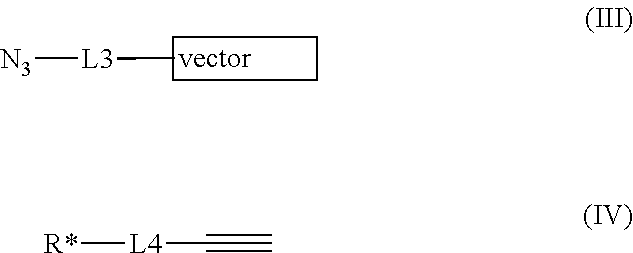
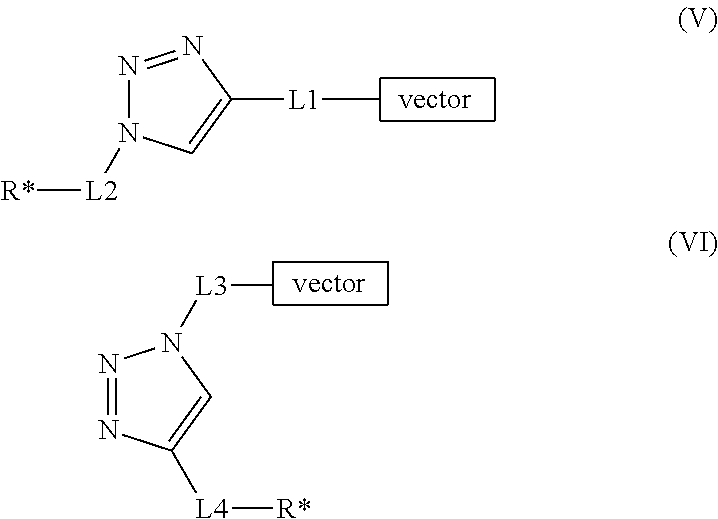
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-Phenyl-4-(tributylstannyl)-1H[1,2,3]triazole (Prophetic example)
[0126]
[0127]Phenylazide can be obtained from Pfaltz & Bauer, Inc., or can be synthesized by the method described in J. Biochem., 179, 397-405 (1979). A solution of tributylethynyl stannane (Sigma Aldrich; 400 mg, 1.27 mmol) in THF (4 ml) is treated with phenylazide (169 mg, 1.27 mmol), copper (I) iodide (90 mg, 0.47 mmol), and triethylamine (256 mg, 2.54 mmol) at room temperature over 48 h. The reaction is then filtered through celite to remove copper (I) iodide and chromatographed on silica in a gradient of 5-20% ethyl acetate in petrol. The second fraction is collected and concentrated in vacuo to give the 1-phenyl-4-(tributylstannyl)-1H[1,2,3]triazole as a colourless oil.
example 2
Preparation of [123I]-1-phenyl-4-iodo-1H[1,2,3]triazole Using Peracetic Acid as the Oxidant (Prophetic example)
[0128]
[0129]To sodium [123I] iodide, received in 5-20 μL 0.05M sodium hydroxide is added ammonium acetate buffer (100 μL pH 4.0, 0.2M), sodium [127I] iodide (10 μL 15 mg / 10 ml 0.01M sodium hydroxide, 1×10−8 moles), peracetic acid (PAA) solution (10 μL 0.01M solution, 1×10−8 moles) and finally, 1 phenyl-4-tributylstannyl-1H[1,2,3]triazole (Example 1; 23 μg, 1×10−7 moles). The reaction mixture is incubated at room temperature for 15 minutes prior to purification by HPLC.
example 3
Preparation of [123I]-1-Phenyl-4-iodo-1H[1,2,3]triazole Using an Iodogen Tube as the Oxidant (Prophetic example)
[0130]
[0131]To an iodogen tube (Thermo Scientific Pierce Protein Research Products), pre-wet with 1 ml pH 7.4, 25 mM sodium phosphate buffer and subsequently decanted is added sodium phosphate buffer (100 pH7.4, 25 mM), and sodium [123I] iodide received in 5-20 μL 0.05M sodium hydroxide. The reaction is allowed to stand at room temperature for 6 minutes with agitation prior to the addition of 1 phenyl-4-tributylstannyl-1H[1,2,3]triazole (Example 1; 23 μg, 1×10−7 moles). The reaction mixture is incubated at room temperature for 15 minutes prior to purification by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Biocompatibility | aaaaa | aaaaa |
| Radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com