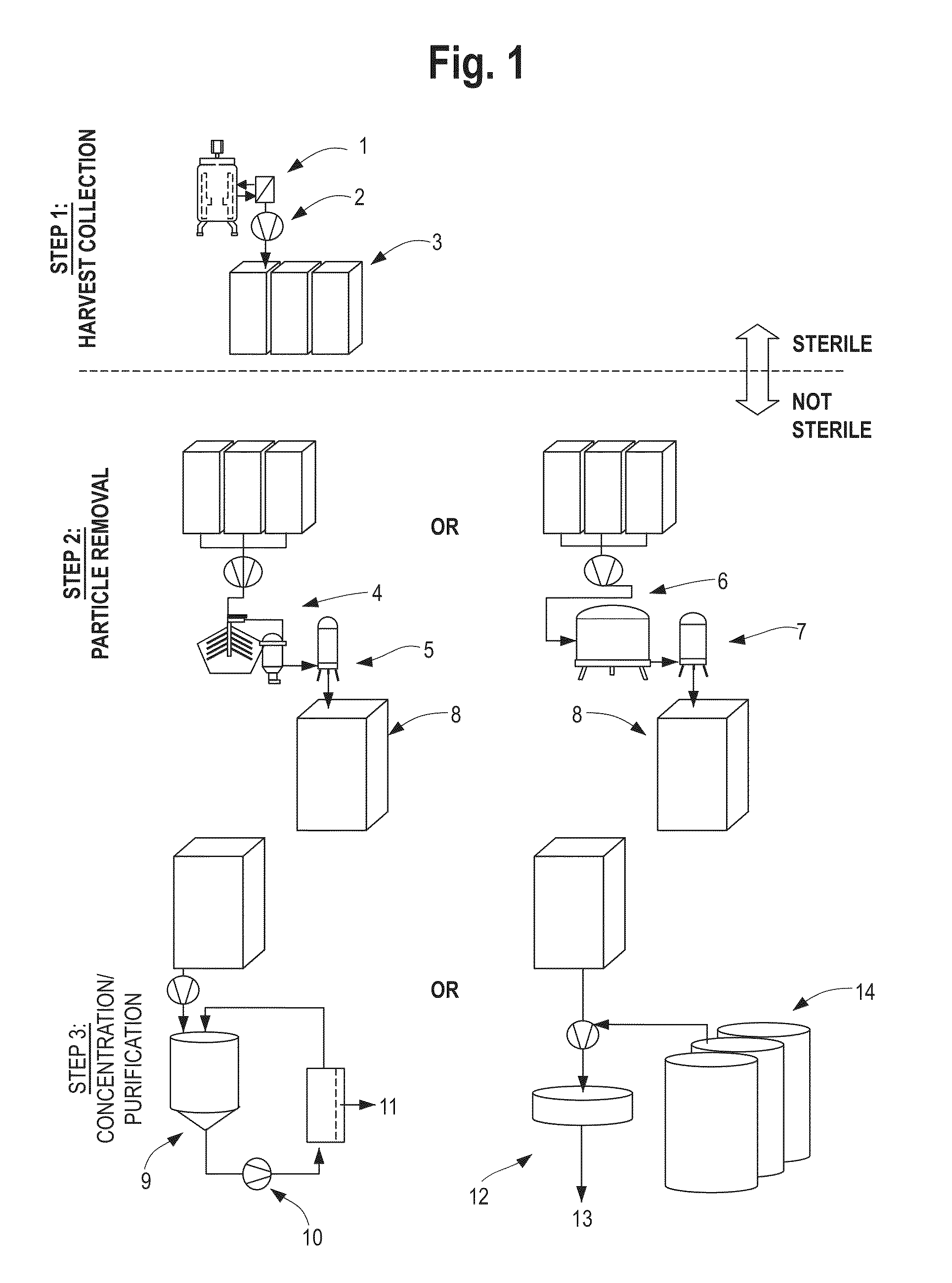
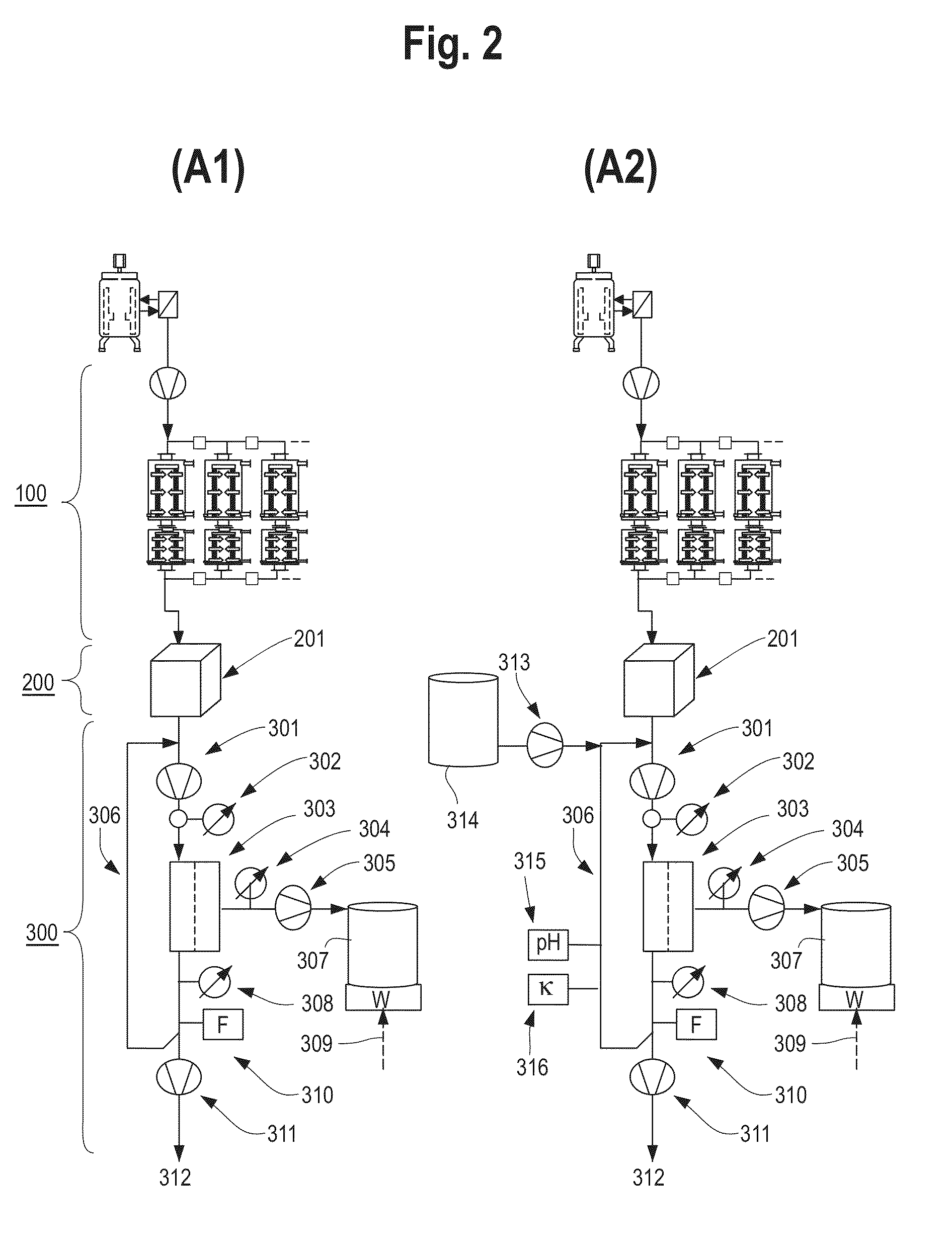
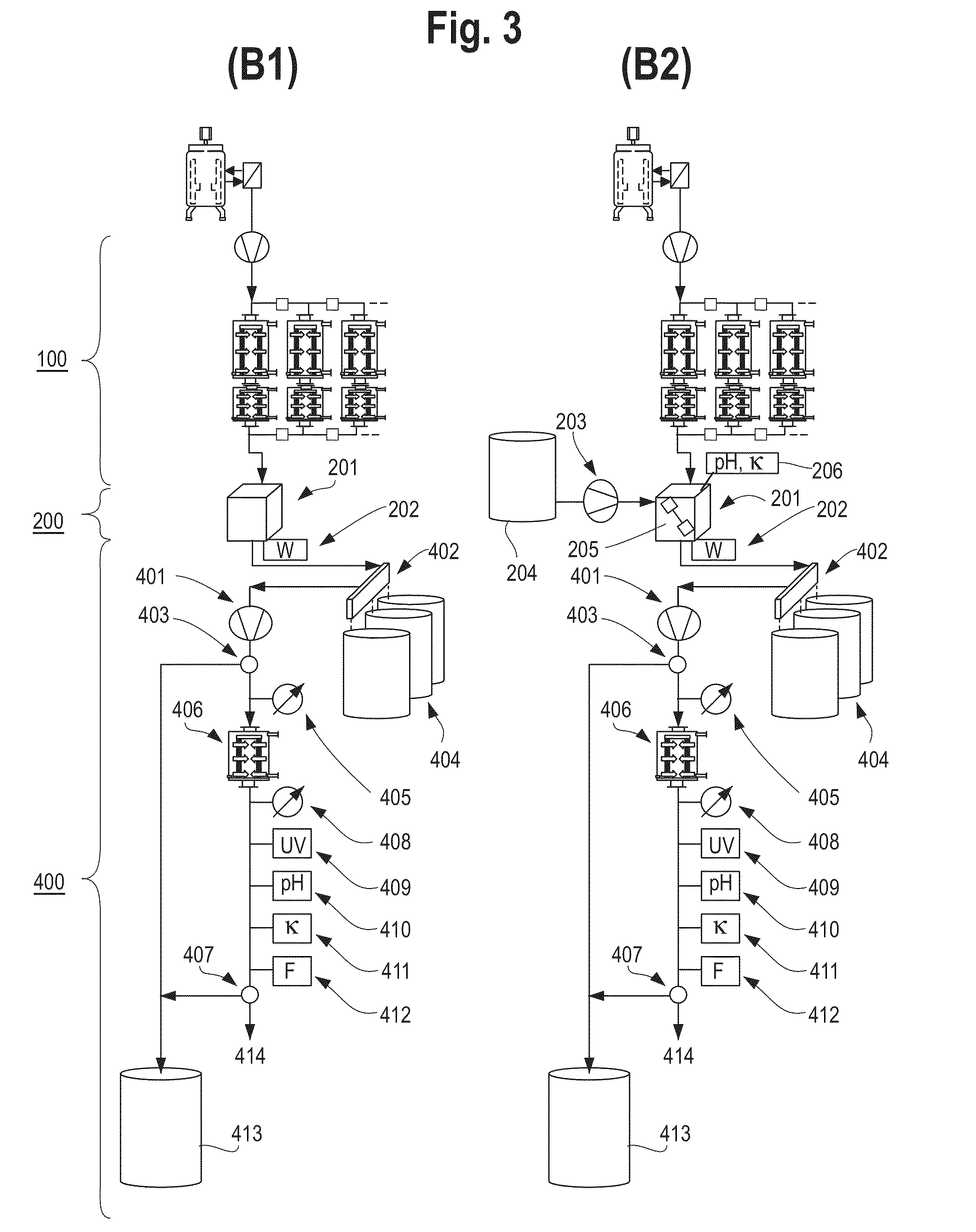
Devices and Methods for Integrated Continuous Manufacturing of Biological Molecules
a technology of biological molecules and manufacturing methods, applied in the field of improved methods and systems for purifying a molecule of interest, can solve the problems of potential quality reduction, yield loss, and inability to meet the requirements of the purification process, and achieve the effect of improving the purification process, and improving the purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Definitions
[0060]Except as expressly defined herein, the terminology used in this application is standard within the art. The following definitions of certain terms are provided herein to assure clarity and definiteness to the meaning of the claims.
[0061]Units, prefixes, and symbols may be denoted in their SI accepted form. Numeric ranges recited herein are inclusive of the numbers defining the range and include and are supportive of each integer within the defined range. Unless otherwise noted, the terms “a” or “an” are to be construed as meaning “at least one of.” The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. All documents, or portions of documents, cited in this application, including but not limited to patents, patent applications, articles, books, and treatises, are hereby expressly incorporated by reference in their entirety for any purpose.
[0062]The term “clarification” and “clarifie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com