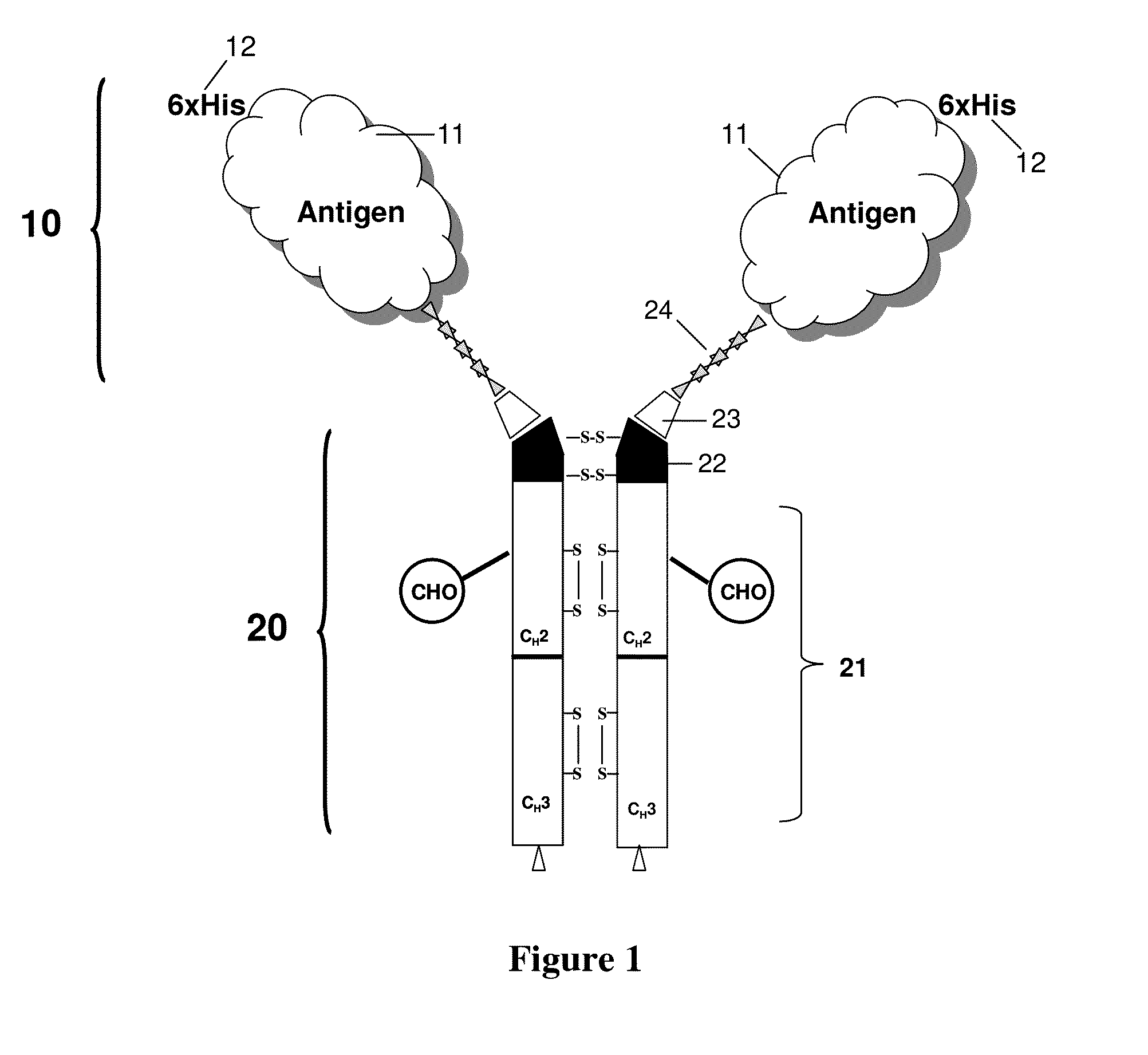
Antigenic compositions and use of same in the targeted delivery of nucleic acids
a technology of antigen composition and nucleic acid, which is applied in the field of compositions for nucleic acid delivery, can solve the problems of few promising and difficult to find suitable delivery methods for these molecules in various applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
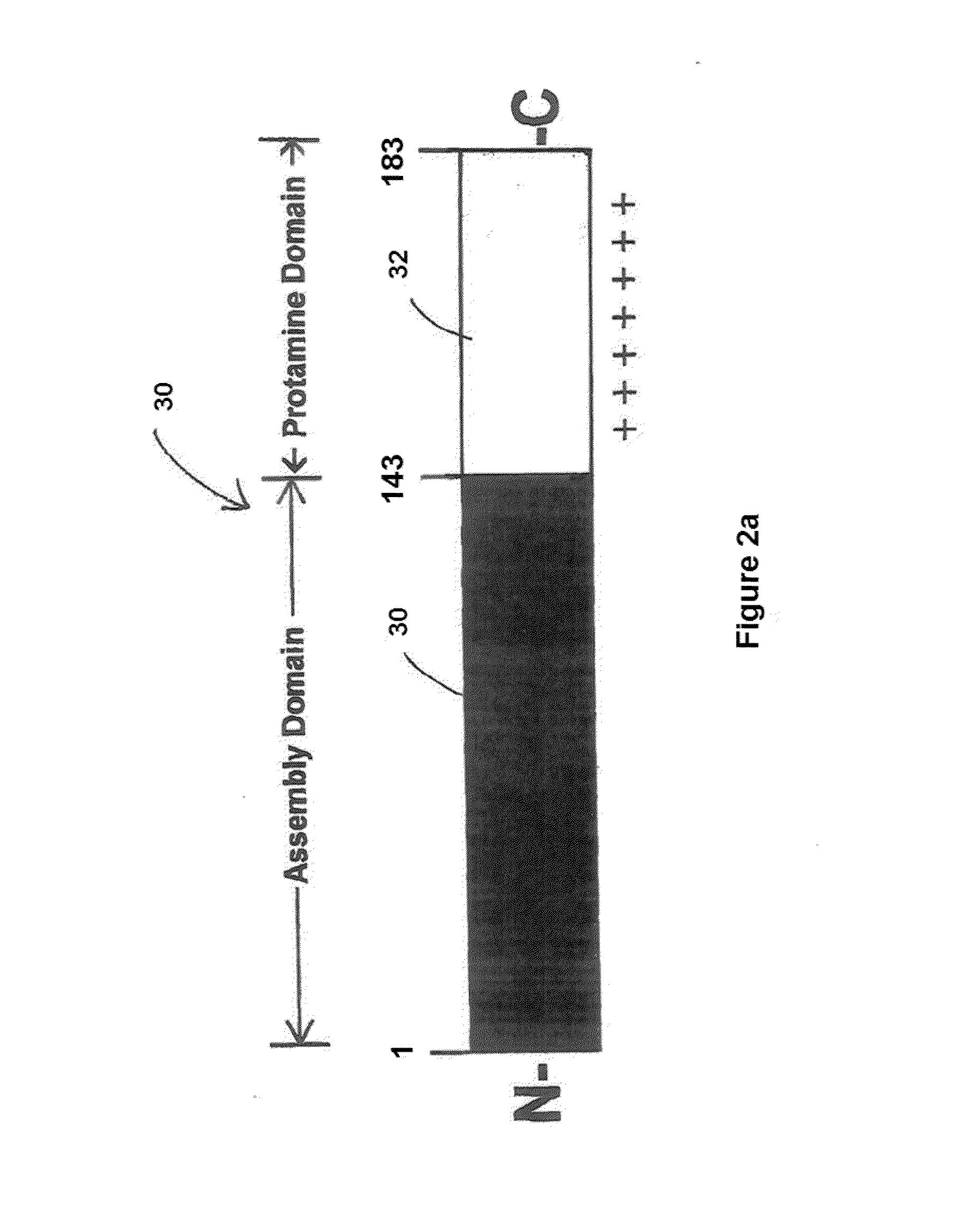
Cloning and Expression of a Chimeric Antigen with a C-Terminal Protamine Tail
[0108]Step 1. Cloning—DNA encoding a target binding domain (TBD) containing a 5′ Not I site and a 3′ Xba I site was produced by PCR using previously generated pFastBacHTa-TBD as template with unique primers that add the respective restriction enzyme sites. The primers used were;
5′ Primer(SEQ ID NO: 8)5′ TGTCATTCTGCGGCCGCAAGGCGGCGGGATCCGTGGACAAGAAAATTGTGCCCAGG 3′3′ Primer(SEQ ID NO: 9)5′ CCGGTCTAGATTCAGCCCAGGAGAGTGGGAGAG 3′.
The PCR fragment was isolated, digested with Not I and Xba I and cloned into a Not I / Xba I digested pFastBacHTa-gp64 plasmid.
[0109]For HBV Core protamine tail, the sequence was obtained by PCR of previously produced plasmid pFastBacHTa HBV Core-TBD as template using primers that add a unique Xba I site to the 5′ end and a unique Hind III site to the 3′ end. The primers used were:
5′ Primer:(SEQ ID NO: 10)5′ CCGGTCTAGAGGAAACTACTGTTGTTAGACGAC 3′and3′ Primer:(SEQ ID NO: 11)5′ GCGCAAGCTTTGACA...
example 2
Visualization of Chimigen Aggregation
[0121]Chimigen® HBV Core Vaccine and Chimigen® HBV S1 / S2 Core Vaccine were visualized using Tapping Mode Atomic Force Microscopy (TM-AFM). The images generated are shown in FIG. 6a-d and 7a-d, respectively. As indicated, the aggregates / nanoparticles formed are of uniform size and ellipsoid shape, having a diameter of 30-40 nm and a height of 2 nm.
example 3
Encapsulation of shRNA Plasmid by Chimigen® S1 / S2 Core Vaccine
[0122]SureSilencing shRNA plasmid was mixed with Chimigen HBV S1 / S2 Core Vaccine under denaturing conditions. After removal of the denaturing conditions, encapsulation was evaluated by DNase treatment and PCR amplification of GFP DNA. The shRNA vector plasmid and results are shown in FIGS. 8a and 8b, respectively. It is noted that both vaccines protected the GFP DNA from DNAse treatment, suggesting that the vaccines are capable of forming encapsulated delivery vehicles around nucleic acids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com