Anode assembly for cathodic protection
a cathodic protection and anode technology, applied in the field of galvanic cathodic protection of steel, can solve the problems of cracking and delamination, destroying the ability of concrete to keep the steel in a passive, non-corrosive state, and contaminated concrete, and achieve the effect of improving the performance and service life of embedded anodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
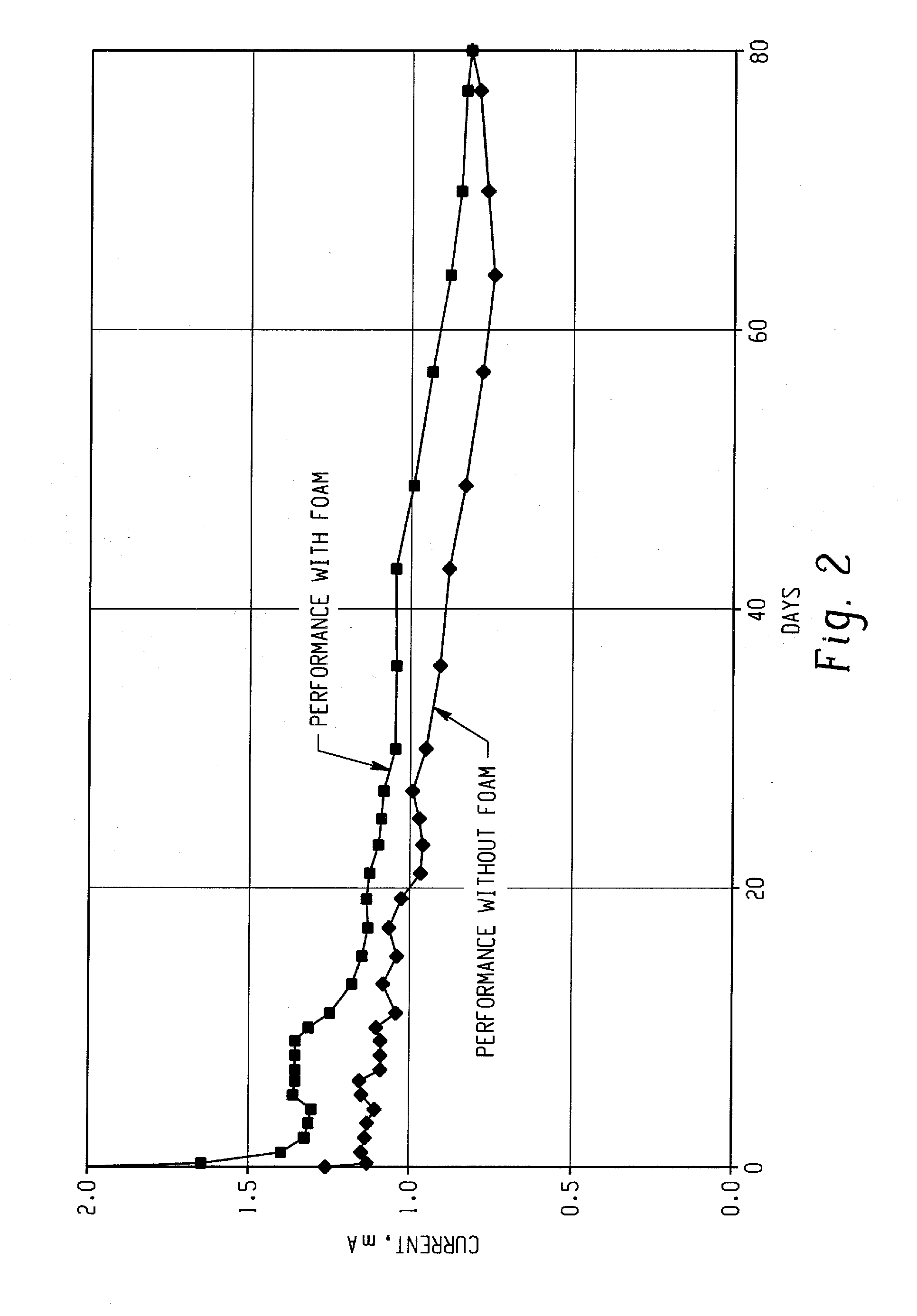
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
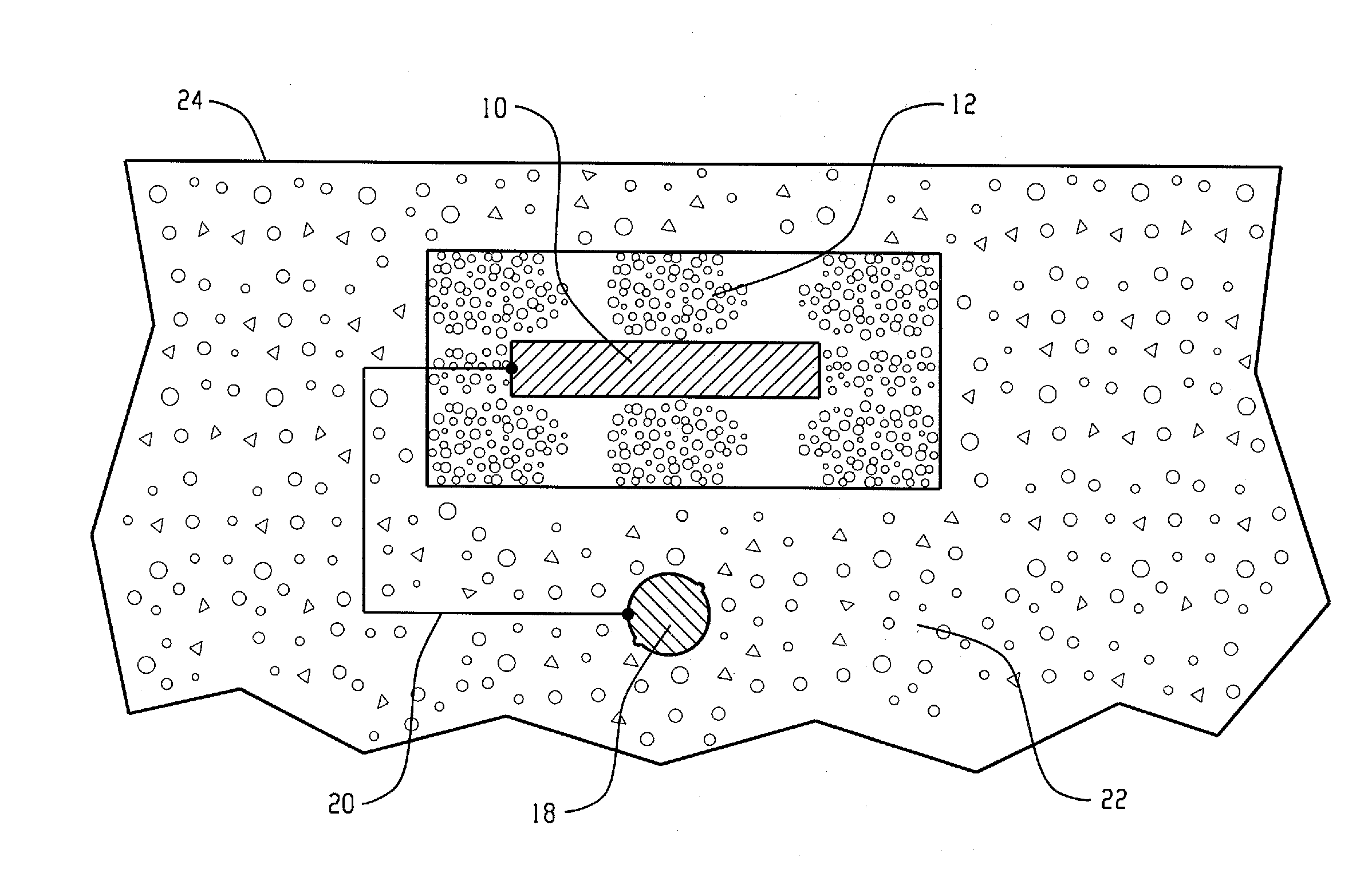
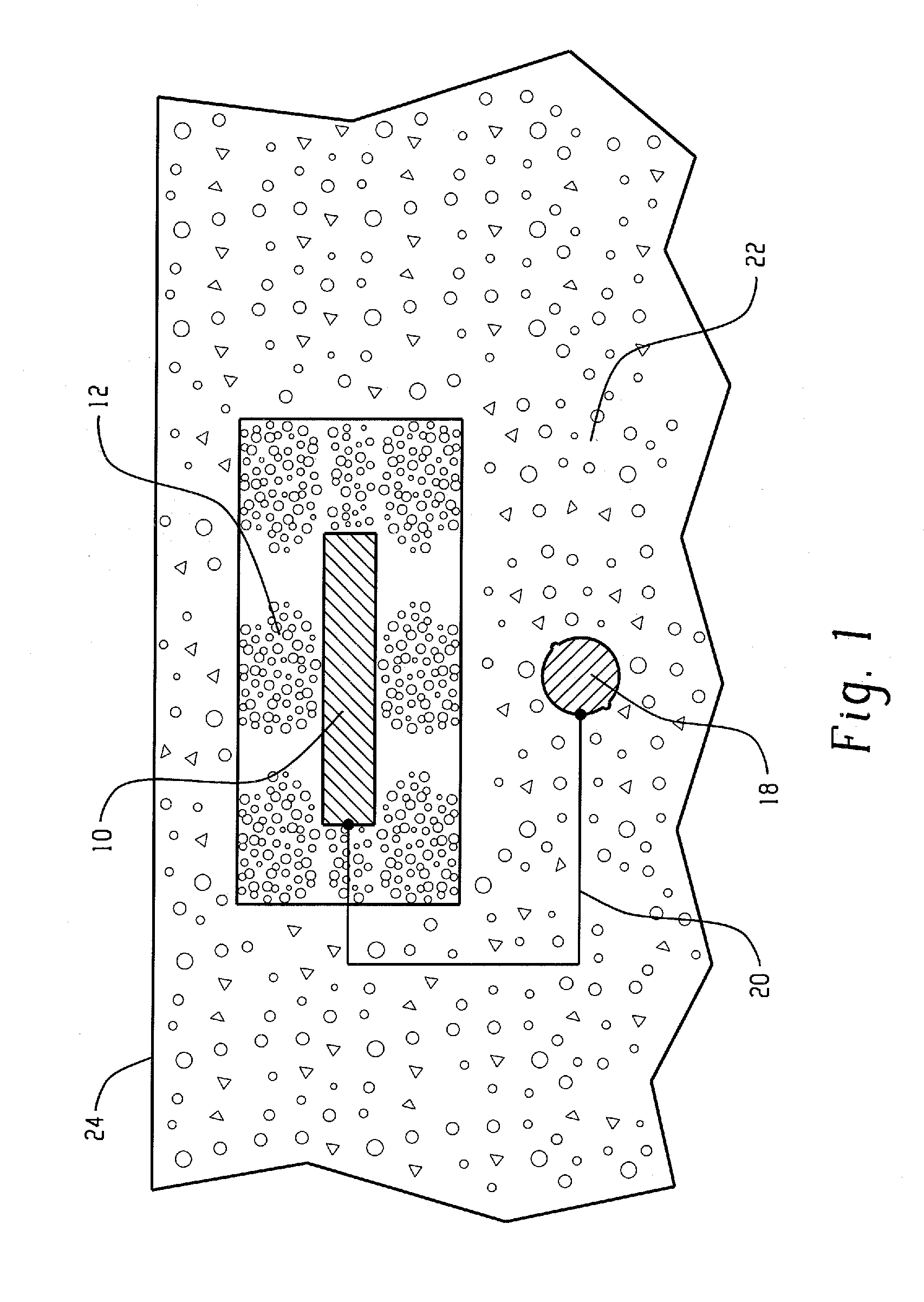
[0029]The present invention relates broadly to all reinforced concrete structures with which cathodic protection systems are useful. Generally, the reinforcing metal in a reinforced concrete structure is carbon steel. However, other ferrous-based metals can also be used.
[0030]The anode assembly of the present invention relates to galvanic cathodic protection (GCP), that is, cathodic protection utilizing anodes consisting of sacrificial metals such as zinc, aluminum, magnesium, or alloys thereof. Of these materials, zinc or zinc alloys are preferred for reasons of efficiency, longevity, driving potential and cost. Sacrificial metals are capable of providing protective current without the use of ancillary power supplies, since the reactions that take place during their use are thermodynamically favored. The sacrificial metal anodes may be of various geometric configurations, such as flat plate, expanded or perforated sheet, or cast shapes of various designs. A preferred configuration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com