Activating matrix for cathodic protection
a technology of activating matrix and cathodic protection, which is applied in the field of galvanic cathodic protection of steel, can solve the problems of cracking and delamination, destroying the ability of concrete to keep the steel in a passive, non-corrosive state, and contaminated concrete, and achieves the effect of improving the performance and service life of embedded anodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0033]A steel reinforced 12×12×4-inch (30.5×30.5×10.2 cm) concrete test block was constructed using concrete with the following mix proportions:
Type 1A Portland cement - 715 lb / yd3Lake sand fine aggregate -1010 lb / yd3No. 8 Marblehead limestone -1830 lb / yd3Water - 285 lb / yd3Chloride (added as NaCl) - 5 lb / yd3Airmix air entrainer (0.95% oz / CWT) -about 6.5% air
[0034]The test block contained about 24 inches (60 cm) of #4 (12 mm dia.) reinforcing bar, or about 0.25 square feet (240 square centimeters) of steel surface area Each test block was cast with two blockouts for two test cells, each blockout forming a circular test cavity about 4 inches (10 cm) in diameter ×2.75 inches (7 cm) deep.
[0035]An anode was first constructed by soldering 40 grams of pure zinc to galvanized tie wires. The zinc was then cast into a mixture containing 65% sand, 15.2% Type III cement, and 19.8% lithium liquid mixture, prepared by combining 40% by volume saturated lithium bromide solution and 60% by volume s...
example 2
[0038]A steel reinforced test block was constructed as in Example 1 above, and a control anode was also prepared as described in Example 1.
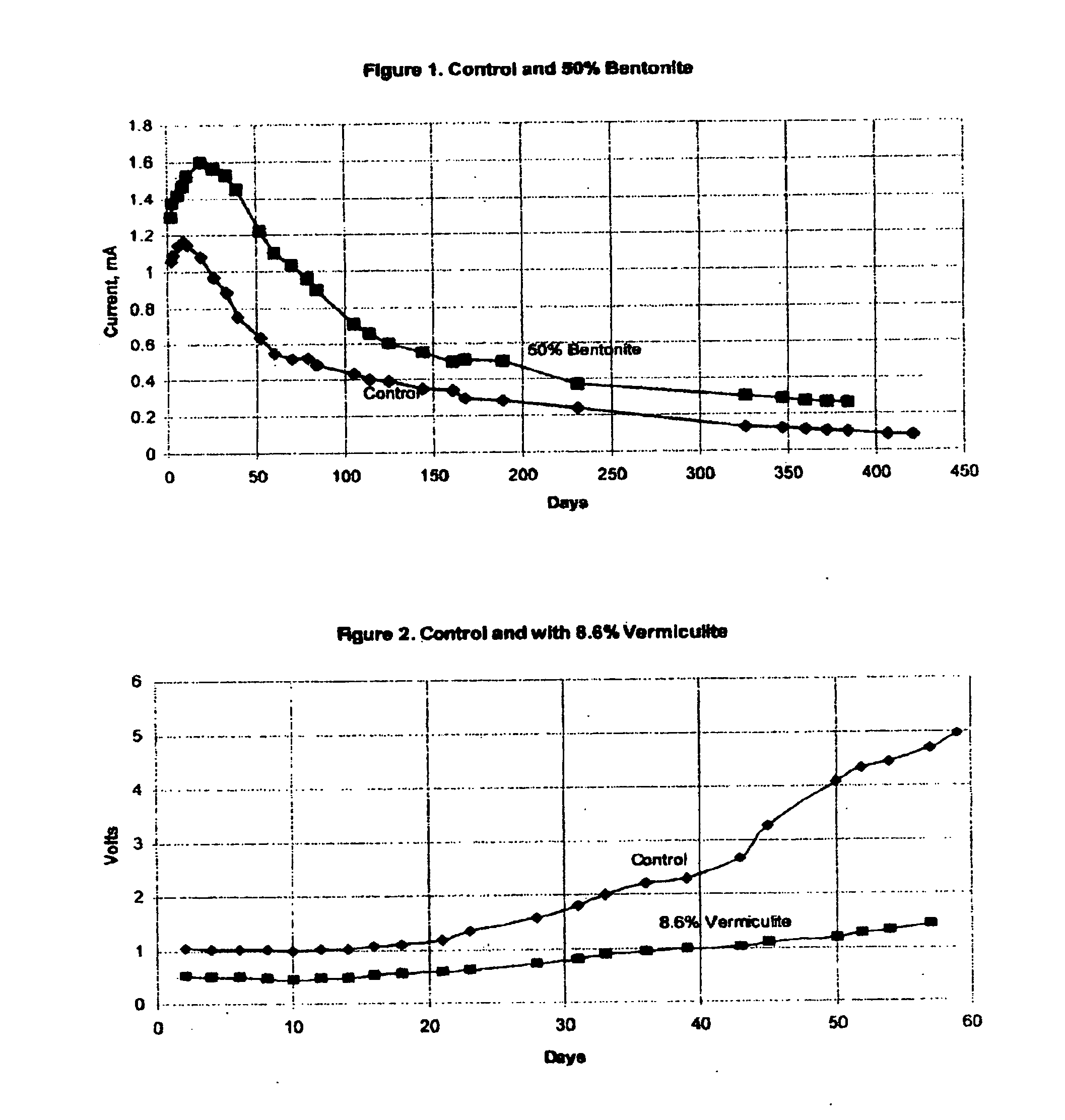
[0039]This anode was subjected to 5 mA of impressed current in constant current mode of operation. In this way, a total charge equivalent to several years of service life can be impressed on the anode in a period of about 60 days. The effectiveness of the anode can be determined by observation of the cell operating voltage. Lower operating voltage indicates that an anode will deliver a higher level of protective current when operated in galvanic mode.
[0040]The operating voltage of the control anode is shown by the line labeled “Control” on FIG. 2. Operating voltage began at about 1.0 volt, and increased to about 5.0 volts after 60 days.
[0041]A second anode was prepared in a similar manner, except that the matrix surrounding the anode contained 8.6% vermiculite by weight. After curing of the mortar surrounding the anode, the anode was placed into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com