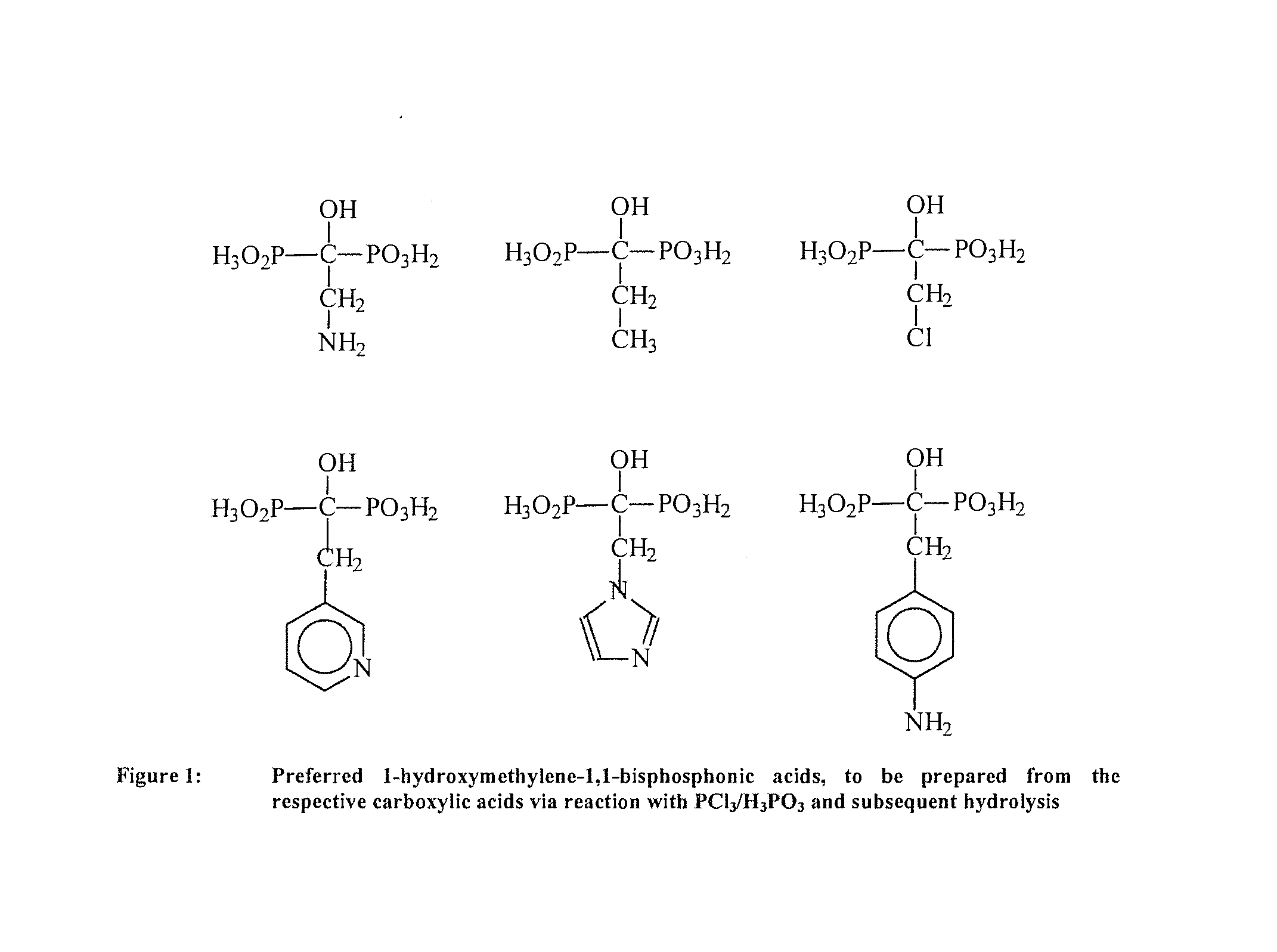
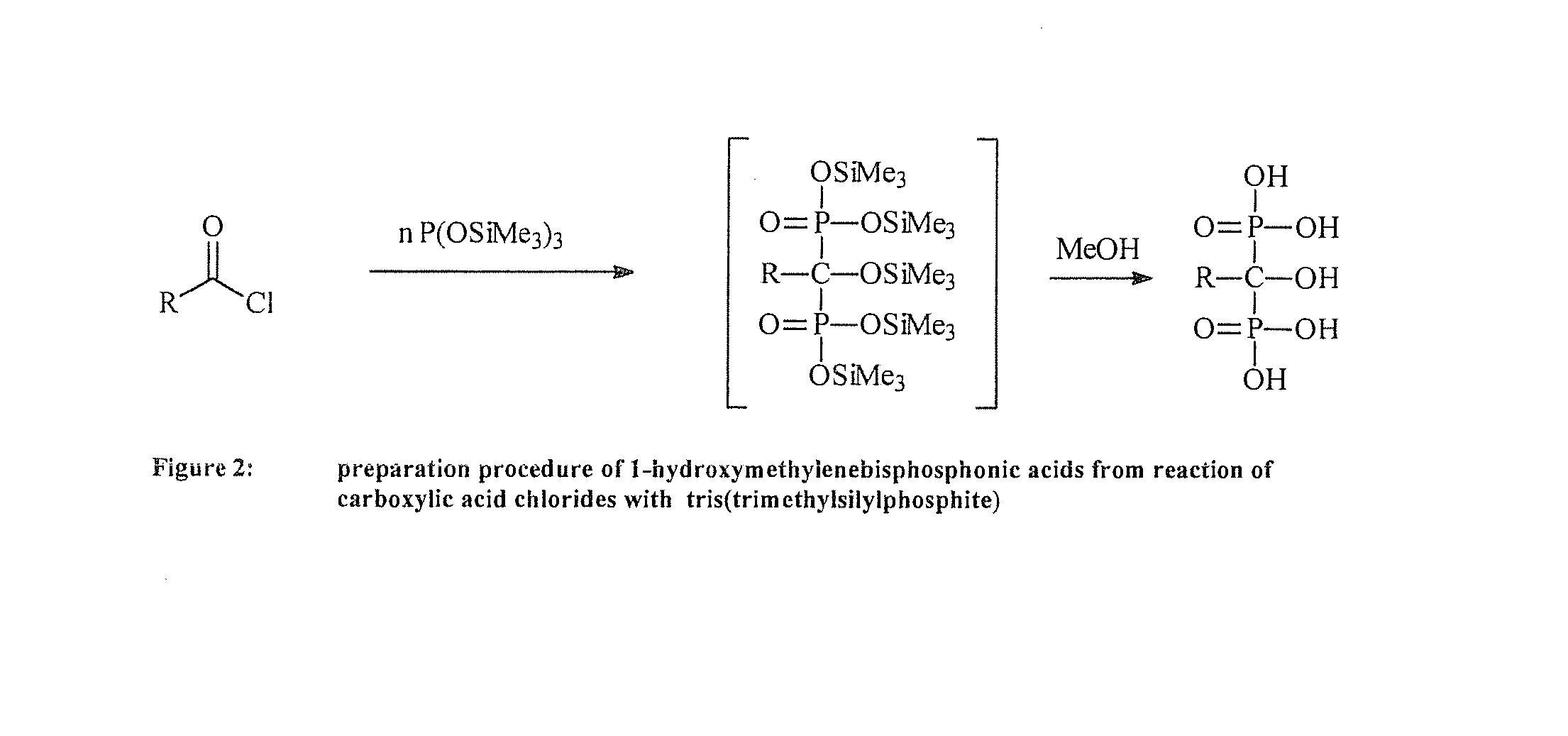
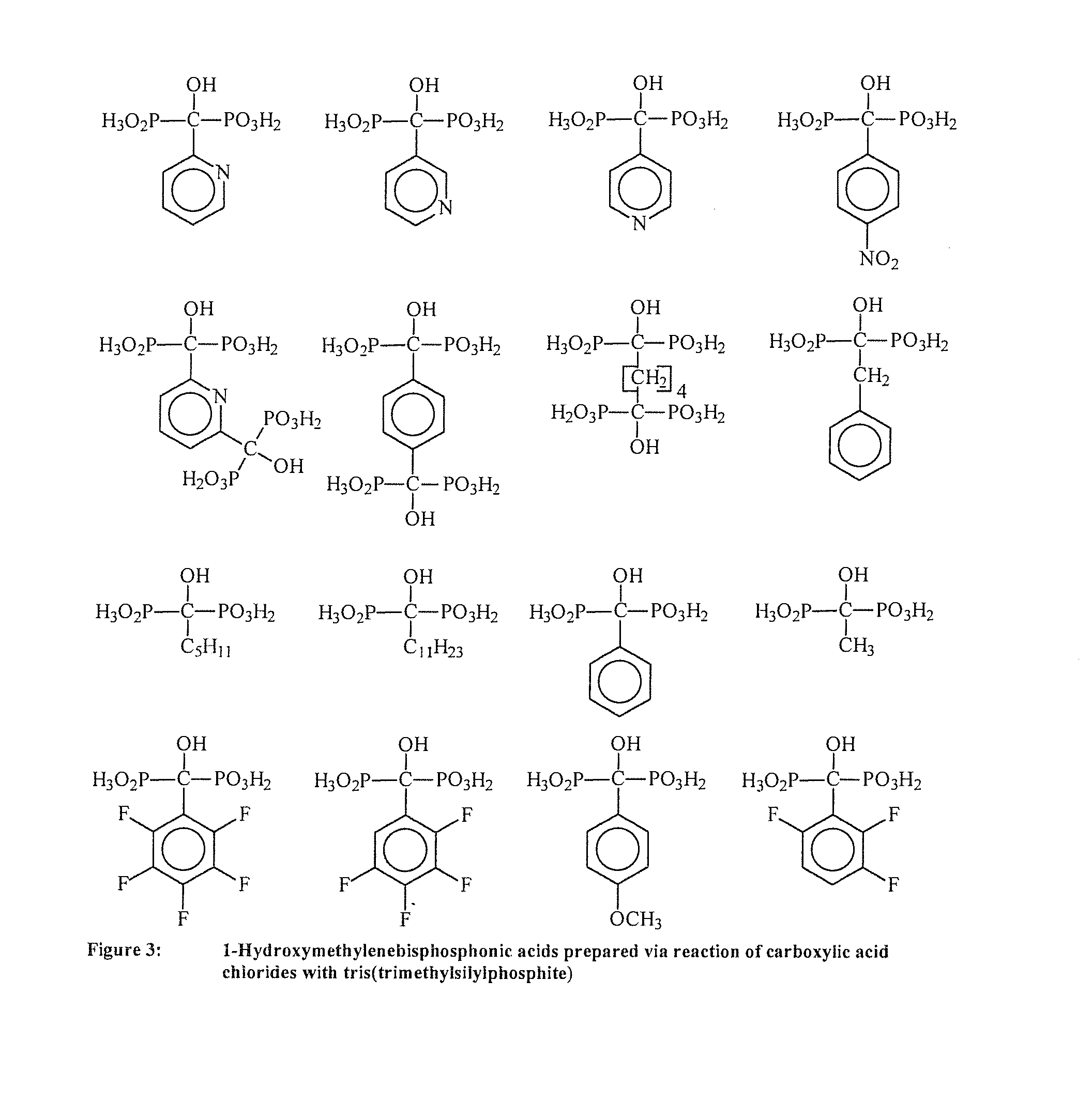
Phosphonic acid-containing blends and phosphonic acid-containing polymers
a technology of phosphonic acid and polymer, which is applied in the direction of cation exchanger materials, sustainable manufacturing/processing, and final product manufacturing, etc., can solve the problems of inability to humidify the membrane, the sulfonated ionomer membrane and the atmospheric pressure is not suitable for us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
application examples
[0097]1. Preparation of an Ionically Cross-Linked Blend from a 1-hydraxymethylene-1,1-bisphosphonic Acid Containing a Pyridine Group and a Sulfonated Arylene Main-Chain Polymer (Universal Procedure)
[0098]3 g of the sulfonated arylene main-chain polymer in the SO3Na form are dissolved in DMSO to a 10% solution. It is dissolved so much of the pyridine-containing 1-hydroxymethylene-1,1-bisphosphonic acid in the Na+ form in DMSO to a 10% solution, that there is 1 sulfonate group per 1 pyridine group. Thereafter the solutions are mixed together. The combined solution is cast onto a glass plate to a thin film with a doctor knife. Then the DMSO is removed via evaporation at temperatures between 50 and 150° C. and, if necessary, low pressure of 800-10 mbar. Then the polymer film is removed under water from the glass plate. The polymer film is posttreated as follows:[0099]1. In 1 to 50% base (alkali base such as NaOH, KOH, LiOH, etc., earth alkali such as Ba(OH)2, Ca(OH)2, aqueous ammonia or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com