Cannabinoids for use in the treatment of neurodegenerative diseases or disorders
a neurodegenerative disease or disorder, cannabis technology, applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of cell death or damage to genetic material, deterioration of the functions controlled by the affected part of the nervous system, cell membrane damage,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effects of Cannabinoids in a Model of Neurodegenerative Disease
Materials and Methods
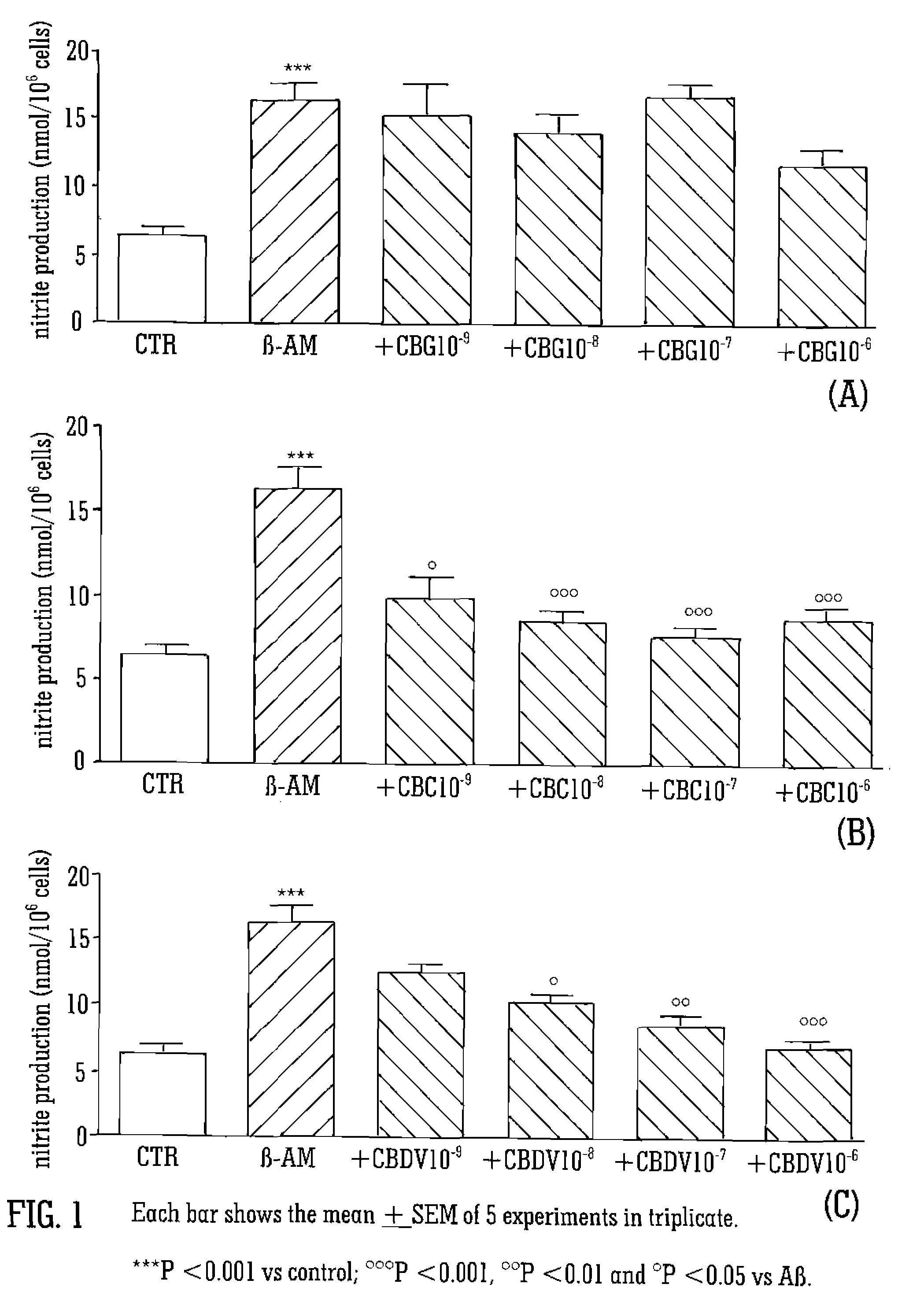
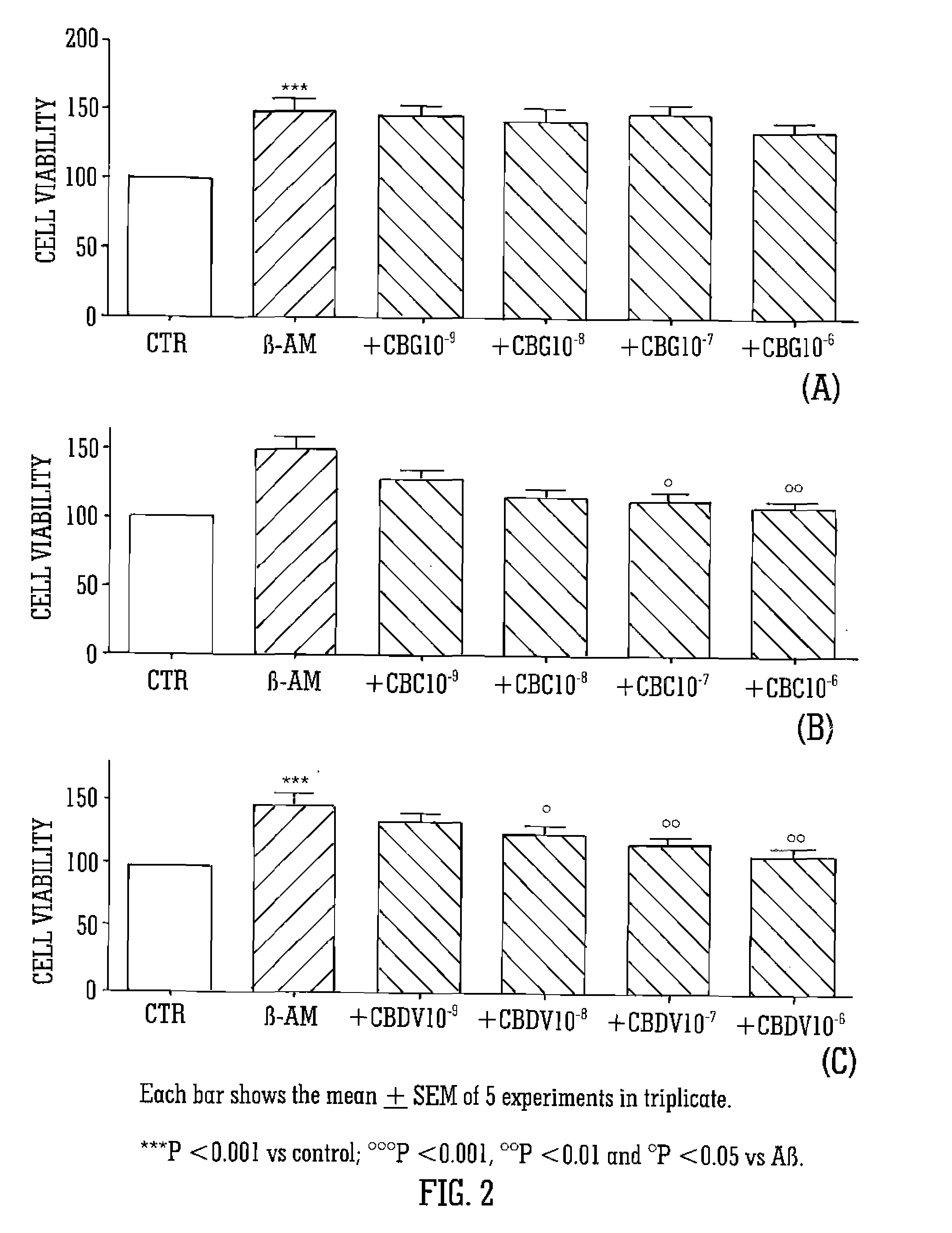
[0068]The cannabinoids tested were CBG, CBC, and CBDV. The cannabinoids were isolated from cannabis plant material and purified. The cannabinoids were tested at a concentration of 10−9 to 10−6 M. The cannabinoids were dissolved in DMSO at the concentration of 10−2M and then diluted in DMEM to their final concentration.
[0069]Glial cells (C6) were cultured in 10% FBS supplemented DMEM. PC12 neuronal cell were cultured in 10% FBS plus 5% HS supplemented DMEM and SHY-5SY neuronal cells were cultured in 10% FBS supplemented RPMI. Neuronal cells were differentiated with retinoic acid. After 24 hours of starvation glial cells, differentiated PC12 and SHY-5SY neuronal cells were treated with the cannabinoids, CBC, CBG and CBDV, in the presence or absence of 1 μg / mL of Aβ (1-42) for the following 24 hours.
[0070]Cell viability was determined using 3(4,5-dimethylthiazol-2yl)2,5-diphenyl-2H-tetrazolium bromi...
example 2
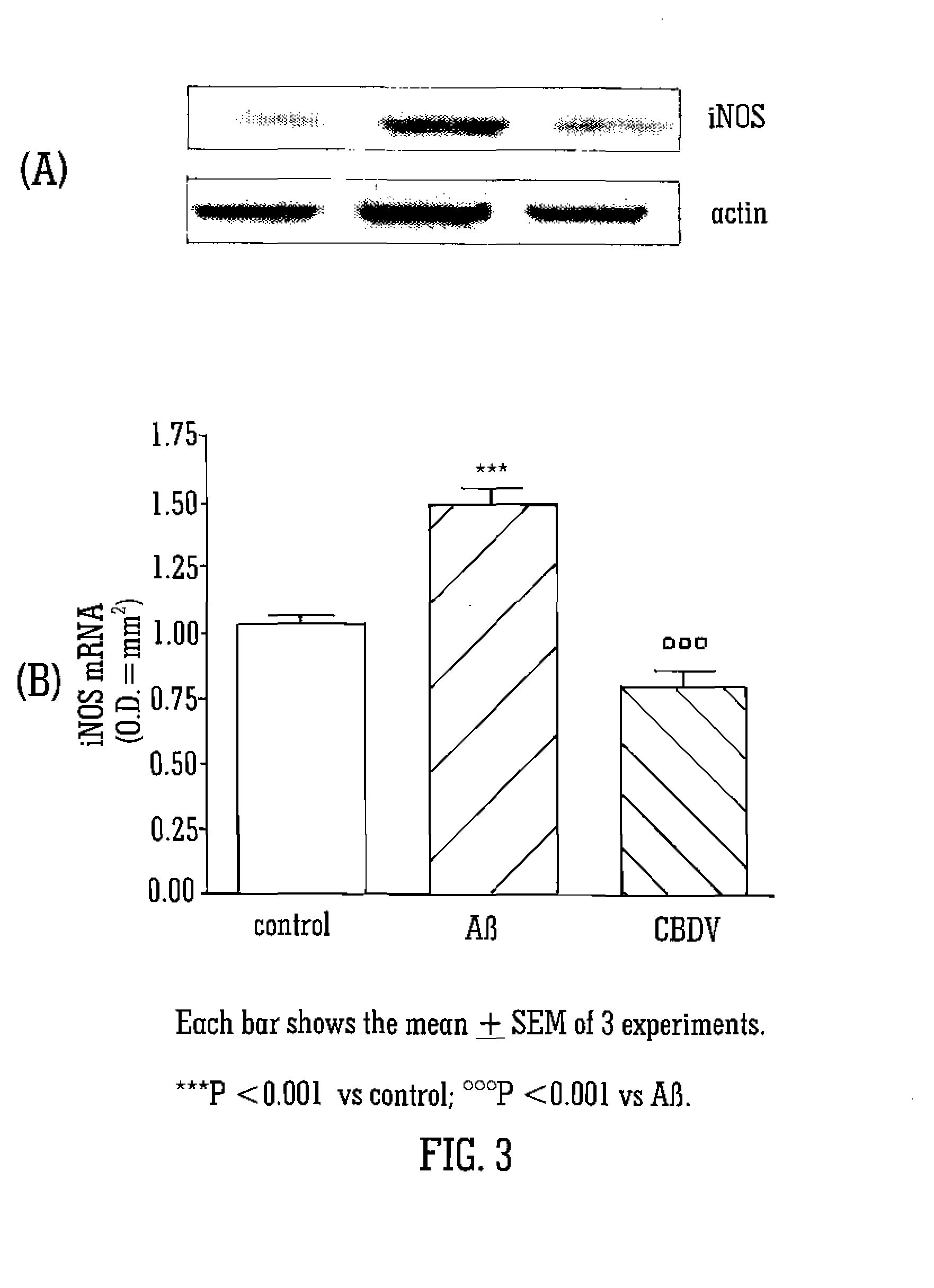
The Neuroprotective Effects of Cannabidivarin (CBDV)
Materials and Methods
[0082]Glial cells (C6) were cultured in 10% FBS supplemented DMEM, and SHY-5SY were cultured in 10% FBS supplemented RPMI. Neuronal cells were differentiated with retinoic acid. After 24 hours of starvation glial cells and SHY-5SY were treated with CBDV (10−7M), CBD (10−7 M), CBDVA (10−7 M), CBDA (10−7 M) and THCVA (10−7 M) in presence or absence of Aβ (1-42) (1 μg / mL) for following 24 hours. All tested compounds were dissolved in DMSO at the concentration of 10−2 M and then diluted in DMEM; DMSO at final dilution that did not show any effects on the parameters under study.
[0083]Cell activation / viability was determined using 3(4,5-dimethylthiazol-2yl)2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, an assay based on the ability of viable cells to convert MTT in formazan salt. Briefly, the cells were plated in 96-well culture plates at the density of 5×103 cells / well and allowed to adhere at 37° C. for 2 hours. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com