Oral composition comprising a TNF antagonist and use thereof
a technology of oral composition and tnf receptor, which is applied in the direction of antibody mimetics/scaffolds, receptors for cytokines/lymphoines/interferons, animals/human proteins, etc., can solve the problem of no oral tnf antagonist or anti-tnf alpha receptor in the market, and achieve the effect of reducing or preventing the effect, constant weight, and weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
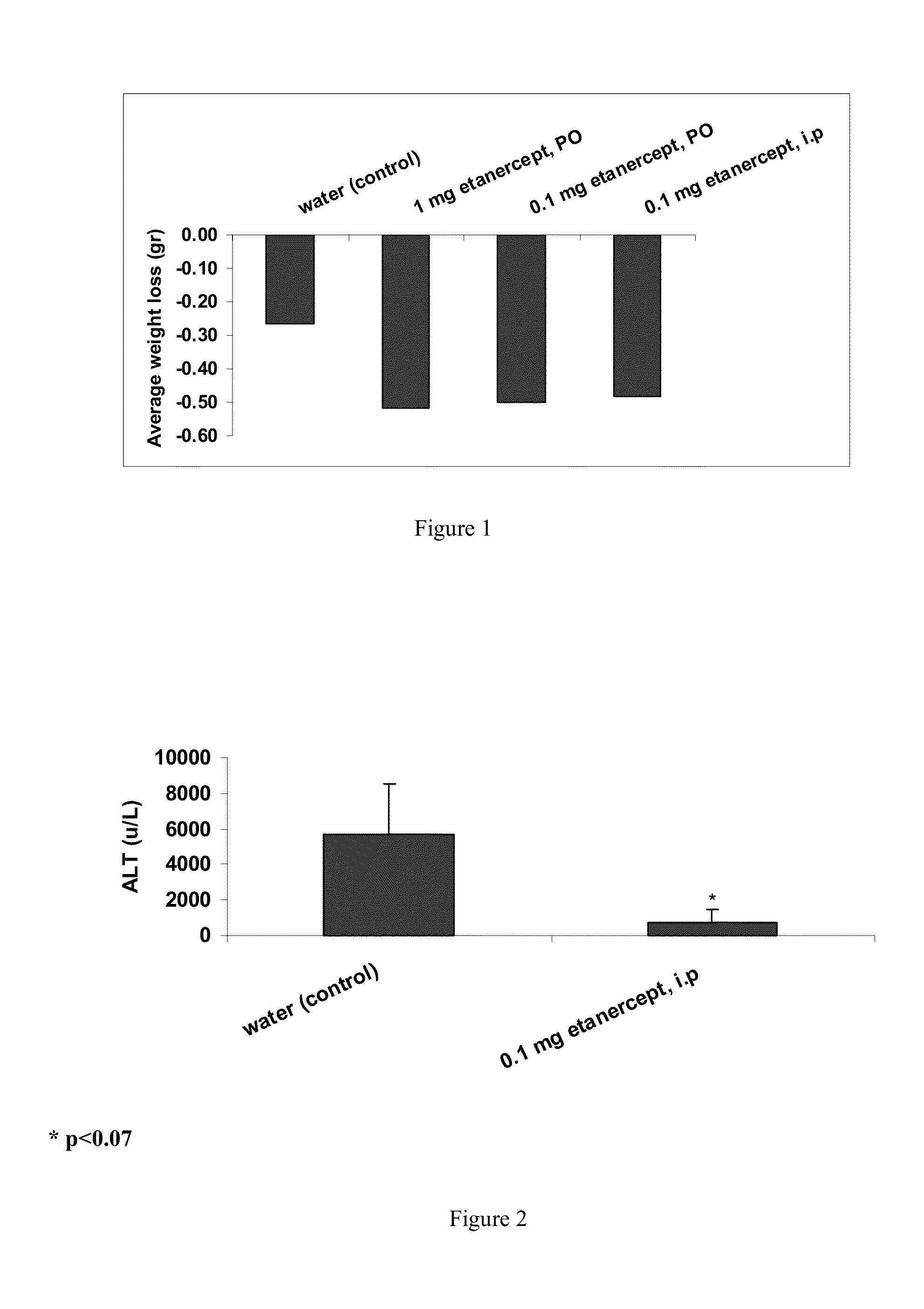
[0086]Measurement of the effect of ENBREL® (ETANERCEPT) on weight loss
Methods:
[0087]Medicine preparation: One ampoule of ETANERCEPT containing 25 mg was dissolved with 1 ml of vehicle (=25 mg / ml, 2500 μg / 1000 μl) according to the manufacturer's instructions.
[0088]Animals: Male C57BL / 6 (B6) mice (12-13 weeks old) were purchased from Harlan Laboratories (Jerusalem, Israel). All mice were maintained in specific pathogen-free conditions. Mice were maintained in the Animal Core of the Hadassah-Hebrew University Medical School. All mice were administered standard laboratory chow and water ad libitum and kept in a 12-hour light / dark cycle.
[0089]Experiment protocol: Four groups (six mice per group) were included in the following experiment.
Group A: Control mice, per oz. administration of 30 μl water.
Group B: Per oz. (PO) administrations of 1 mg of ETANERCEPT.
Group C: PO administrations of 0.1 mg of ETANERCEPT.
Group D: i.p administrations of 0.1 mg of ETANERCEPT.
[0090]Treatment was for six c...
example 2
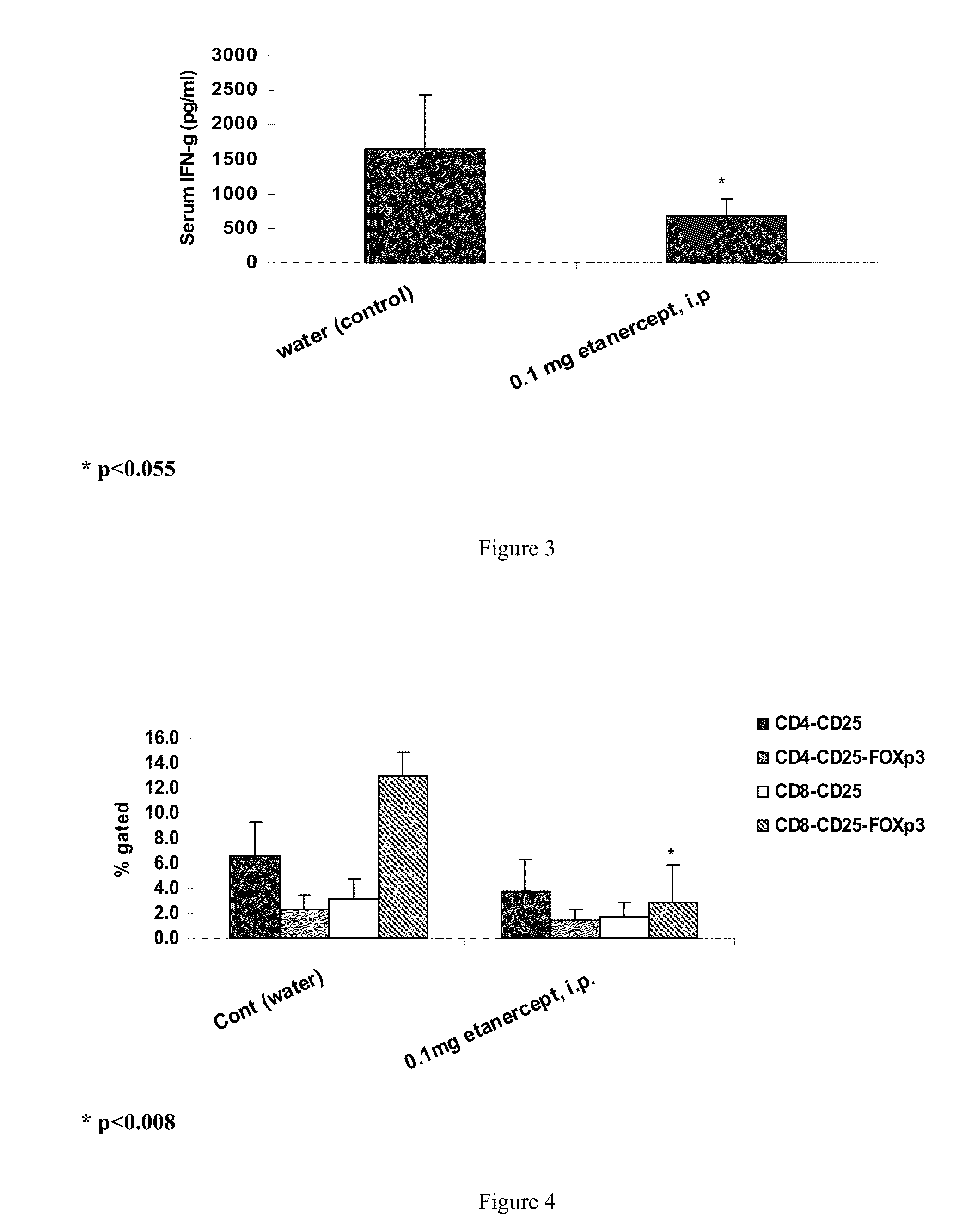
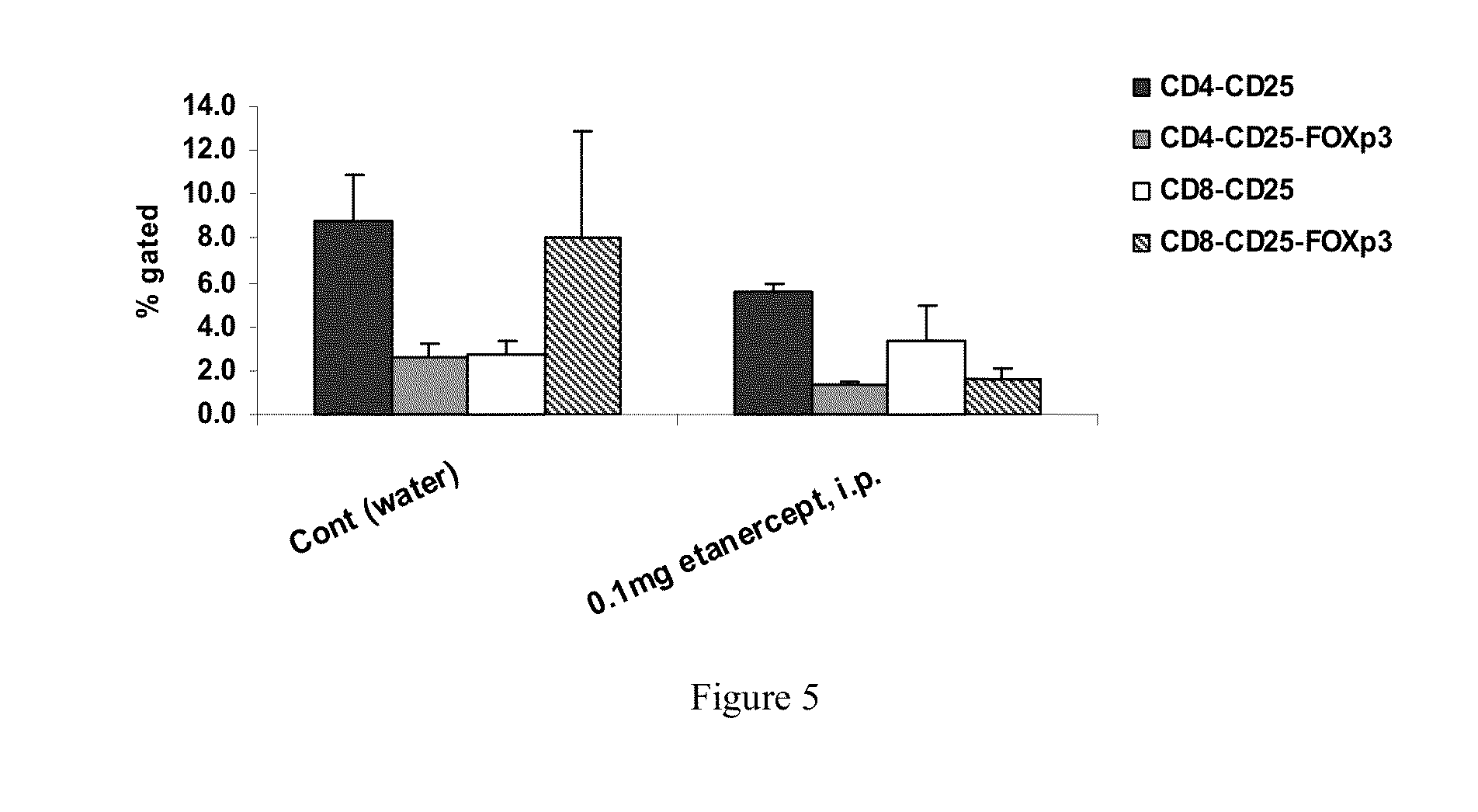
Effect of ENBREL® (ETANERCEPT) in Concanvalin A (ConA) Model
Introduction:
[0092]Several animal models mimicking human liver injury are known to be applied for exploring the immunopathogenesis in liver diseases. However, an acceptable in vivo model for hepatitis C virus HCV does not exist, as HCV is a non cytopathic virus and its liver damage is immune mediated.
[0093]ConA induced hepatitis. The Concanvalin A (ConA) model is a widely utilized mouse model that mimics many aspects of human autoimmune hepatitis. ConA is a bean lectin, which when injected intravenously to mice, induces activation of lymphocytes in the liver. ConA induces massive liver necrosis in mice, simultaneously with the lymphocyte infiltration in the liver, high level of apoptotic hepatocytes and elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The activated lymphocytes in the liver injury were later on confirmed to be Natural Killer T (NKT) cells. Together with Kupffer cells, NKT c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| weight loss | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com