Human disc tissue
a technology of disc tissue and stem cells, which is applied in the field of human disc tissue, can solve the problems of neck and lower back pain from degeneration permanent disruption of the joint, and herniation or protrusion of the disc, so as to prevent, inhibit, or decrease the likelihood of damage or disease of the spinal disc joint, and prevent, inhibit or decrease the likelihood of damage or disease of the cartilage-containing join
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Discotek Culture Platform
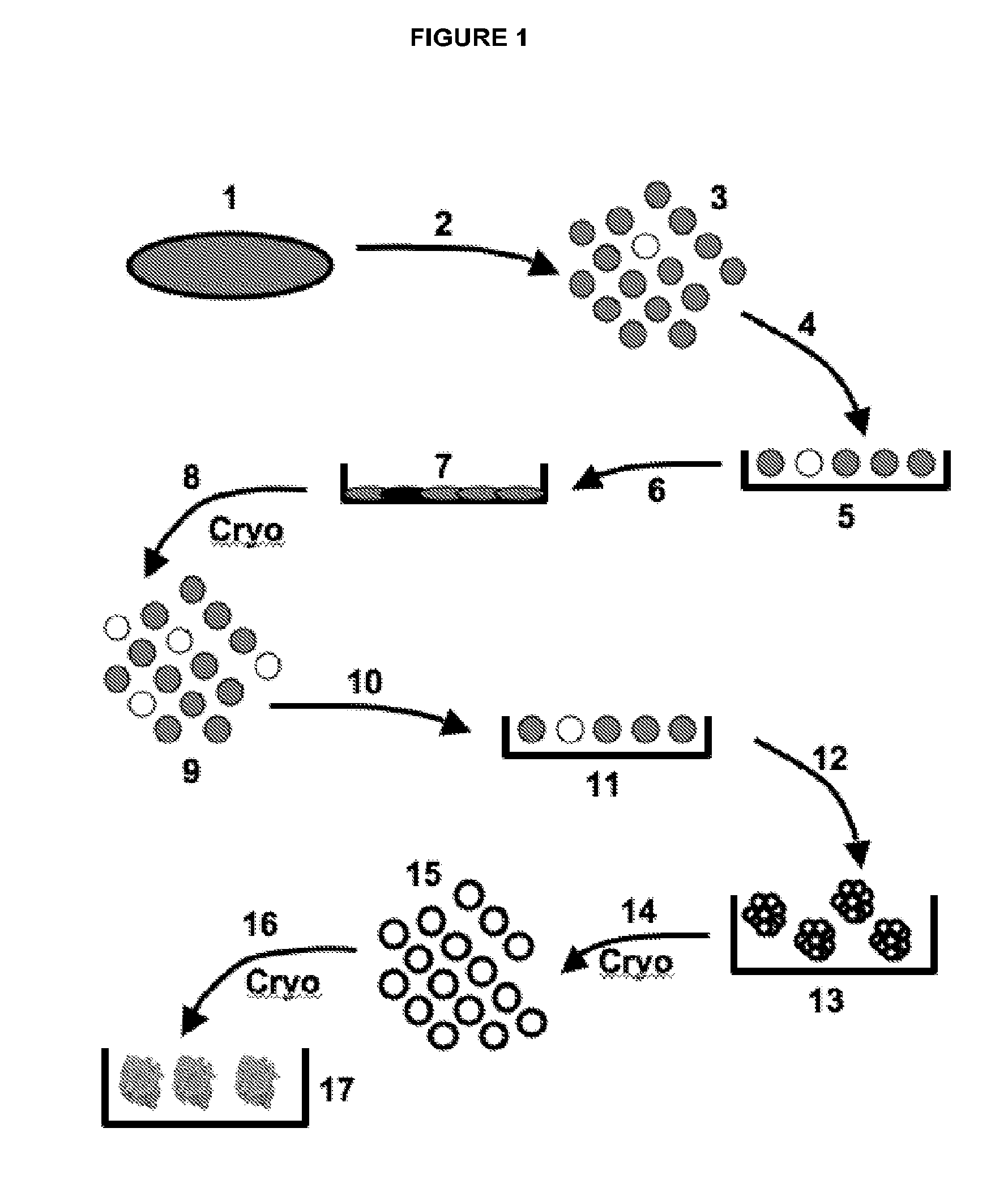
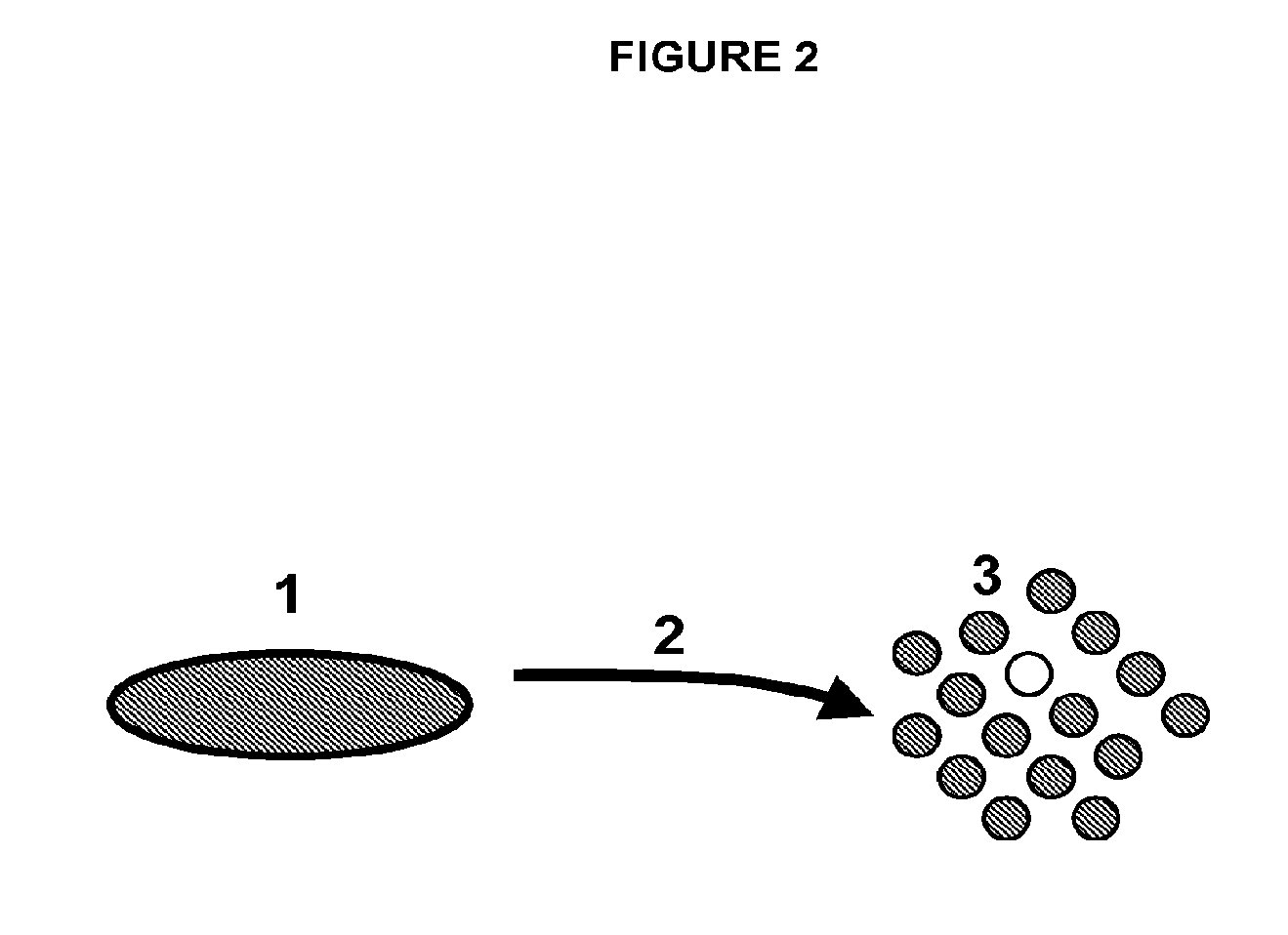
[0315]In one embodiment, the Discotek Culture Platform comprises the steps of (1) preparing a single cell suspension from a disc tissue specimen (FIG. 1, reference numerals 1-3), (2) the Attachment Culture Method (FIG. 1, reference numerals 4-7), (3) the Discosphere Culture Method (reference numerals 8-13), and (4) the Cluster Culture Method (FIG. 1, reference numerals 14-17).
[0316]Disc tissue was procured from a human patient during surgery, or from an experimental animal model (FIGS. 1-2, reference number 1). The tissue was processed by dissection and then enzymatically digested overnight for 24 hours at 37° C. in Collagenase II (FIGS. 1-2, reference number 2). The result was a single cell suspension of all cells that comprise the disc tissue that can be plated into the subsequent culture steps (FIGS. 1-2, reference number 3).
[0317]The single cell suspension was plated into the attachment culture (reference number 4), in which the plates were coated with g...
example 2
Preparation of Single Cell Suspensions
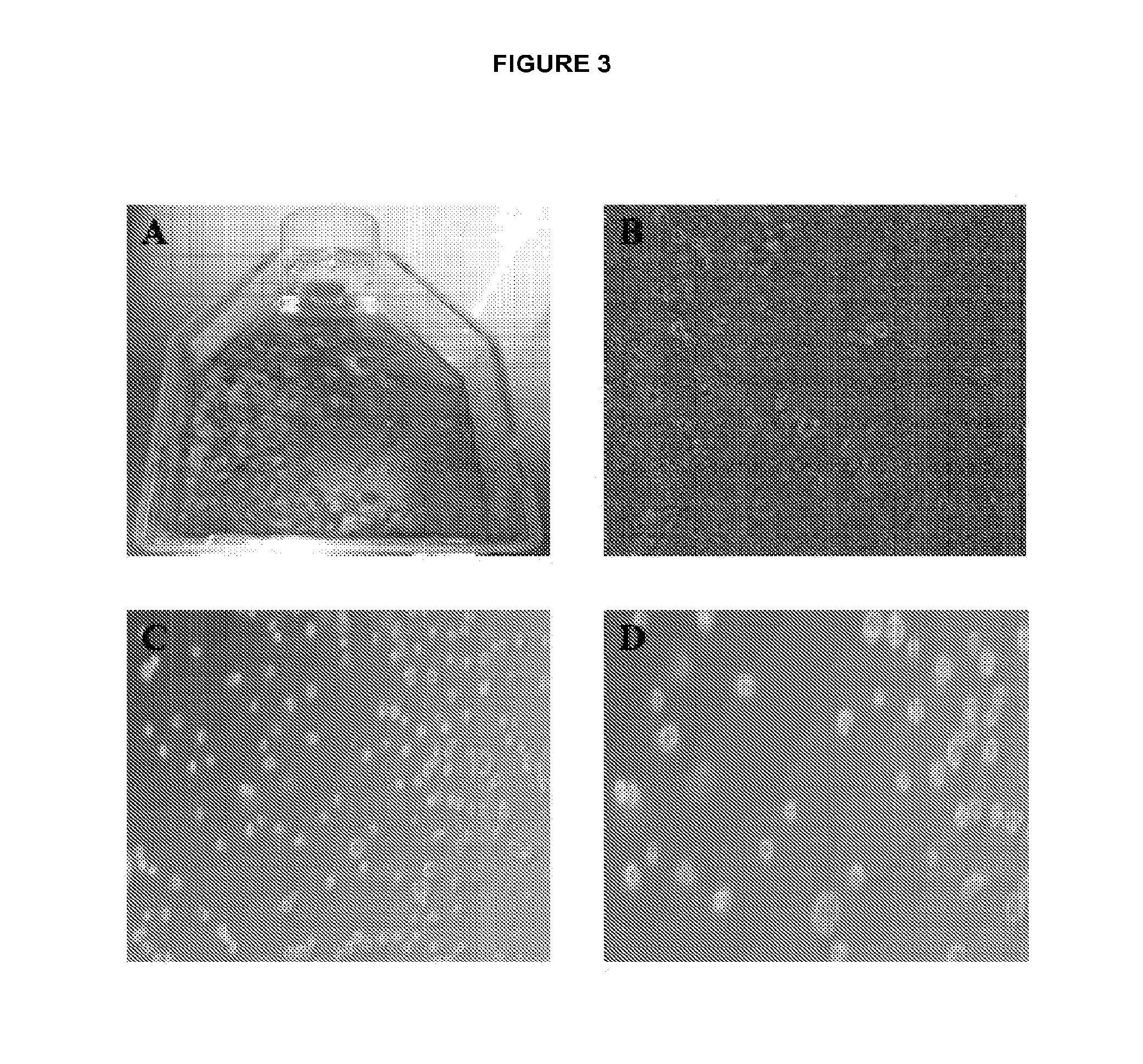
[0320]Human disc tissues were dissected into smaller pieces, and washed thoroughly of blood and other tissue contaminants (FIG. 3A). The dissected fragments were placed in media with Collagenase II at 300 units / ml and incubated for 24 hours at 37° C. (FIG. 3B). A large amount of debris was present that made counting cells difficult and was removed with the washing step. A cell strainer was not used because of the routine presence of cellular clusters that are potentially notochordal clusters that do not break up without further trypsinization and trituration. It was thought that these cell clusters were a critical source of notochordal cells and that they resist digestion due to their extensive extracellular matrix. A single cell suspension was the product of this method (FIGS. 3C-D). This method does not use Trypsin for prolonged digestion, and does not use mechanical disassociation of tissues, to protect the fragile disc stem cell population. ...
example 3
Attachment Culture Method
[0322]The single cell suspension was plated into the Attachment Culture Method (FIG. 5), in which the plates were coated with gelatin, causing the single cell suspension to attach as it settles to the culture flask floor and contacts the surface (FIGS. 1 and 5, reference numerals 4-5). The cultures then grew efficiently as a monolayer reaching confluency in 4-7 days when the starting disc tissue material was derived from young healthy disc tissue specimens. All species and even degenerated tissues grow well with this culture method, but the rate of growth varies and is delayed with tissues from older patients or animals, and degenerated tissues, and certain species (i.e., rat, rabbit, neonatal porcine, young healthy human tissue), as compared to specimens derived from humans that are older, degenerated tissues from any species, or certain other species that are more acellular generally speaking (i.e., adult porcine, cow, etc.) When the cells were confluent, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com