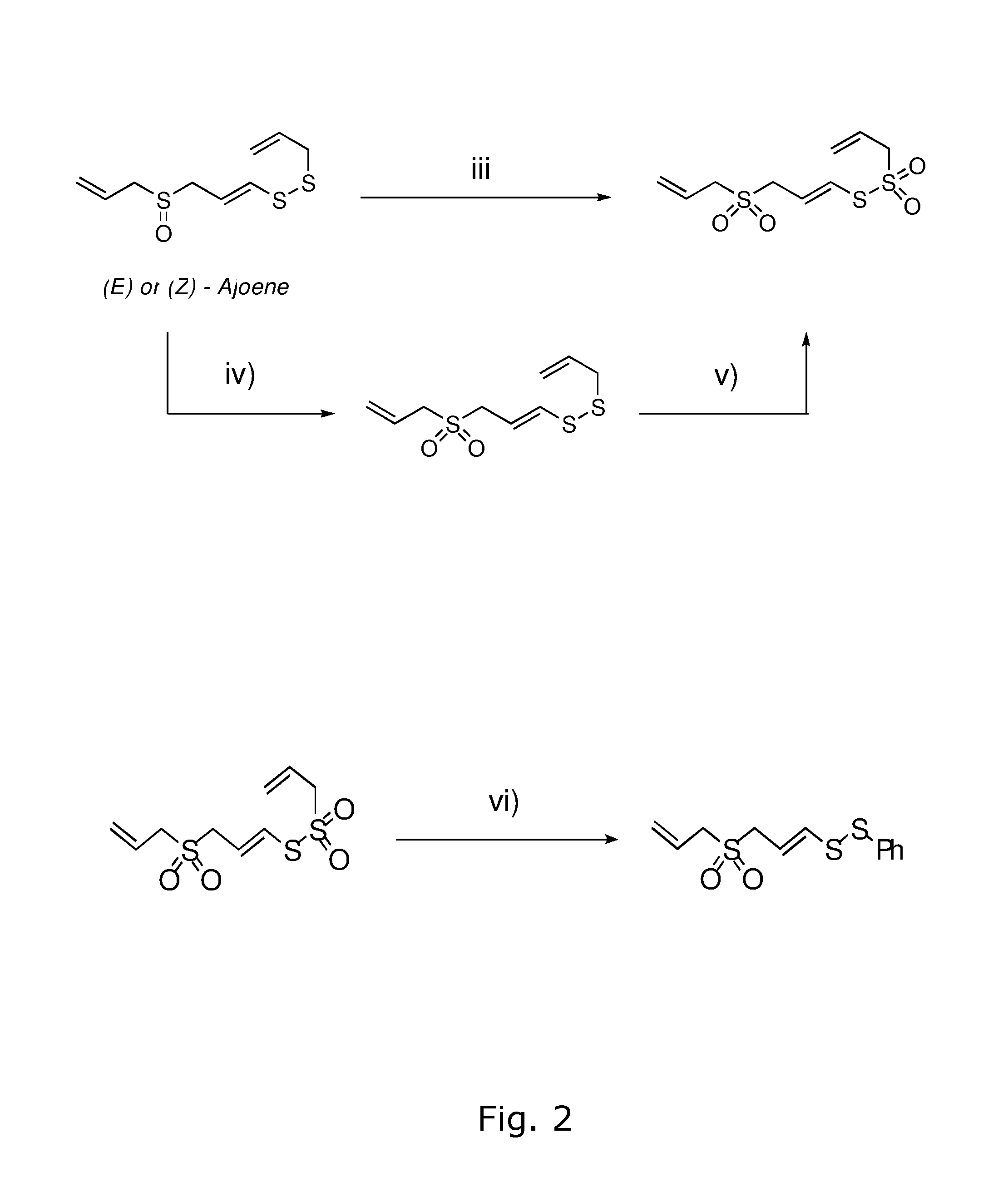
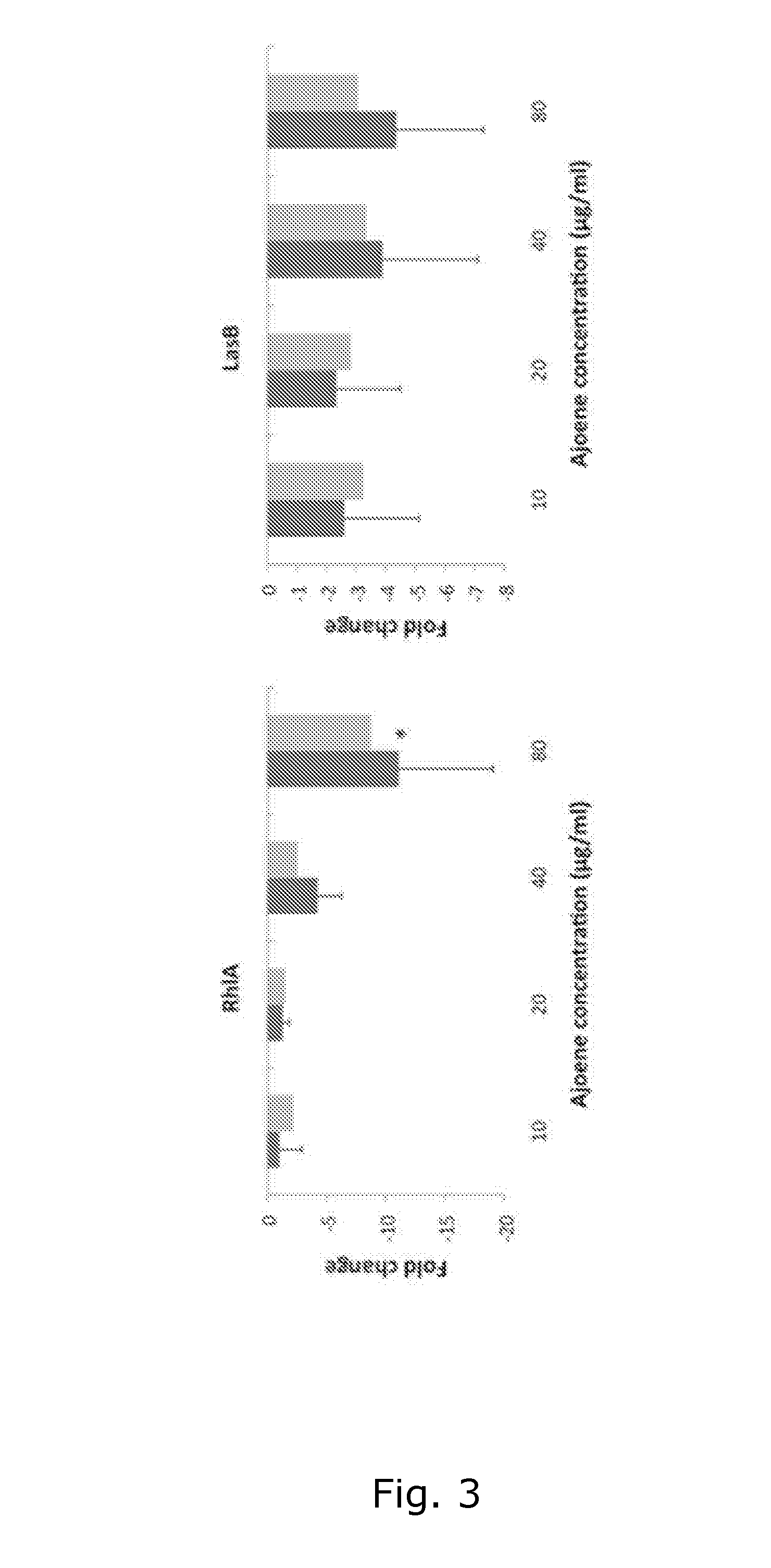
Process for the manufacture of ajoene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
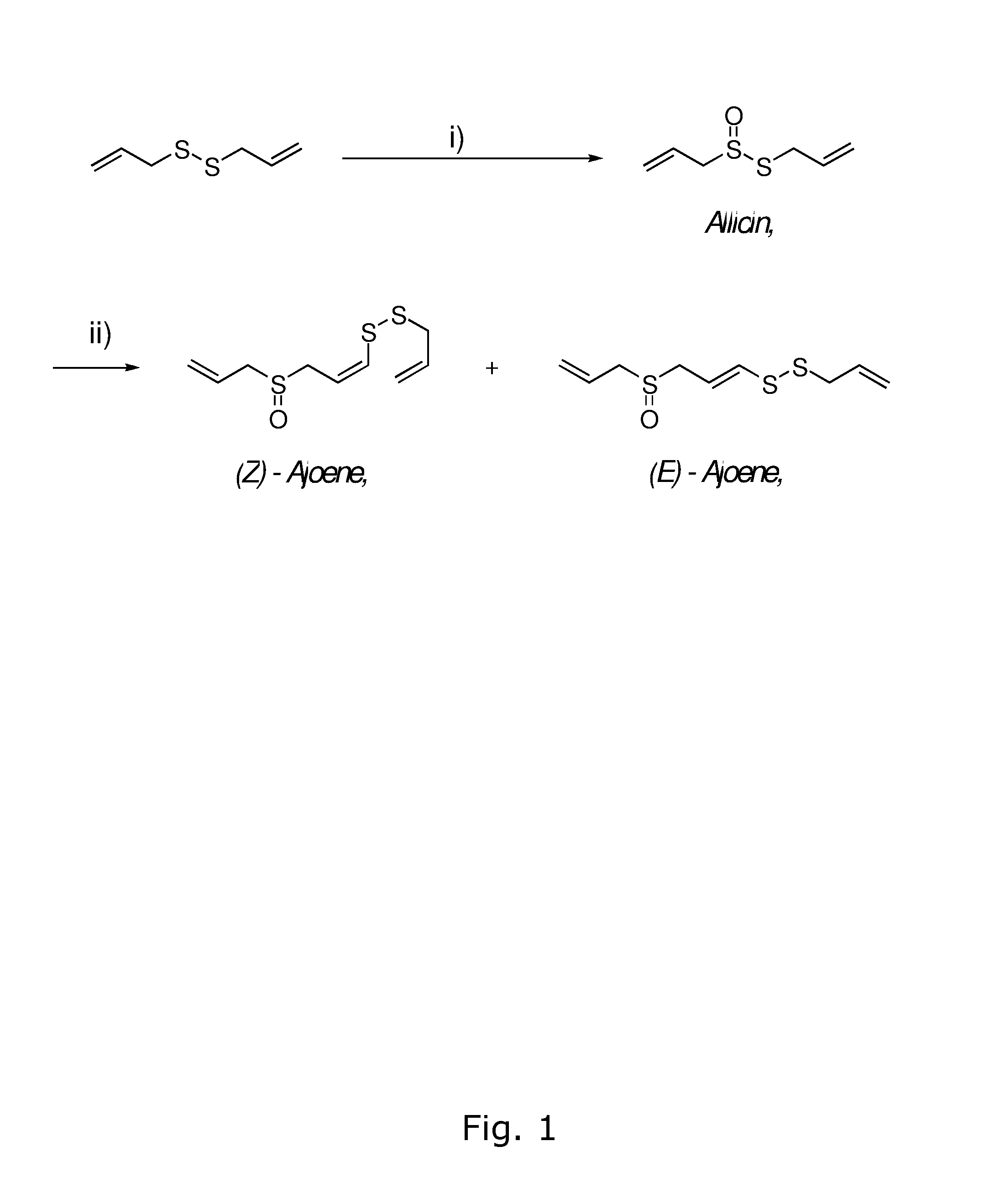
Synthesis of Compound (3), Allicin
[0066]An acetone solution of DMDO (31 mL, 0.07M, 2.2 mmol) was added dropwise over 15 min to compound (2), diallyldisulphide (0.29 g, 2.0 mmol) in acetone (1 mL) at −50° C. under argon. The resulting light yellow reaction mixture was allowed to stir for 30 min while slowly warming to −20° C. The resulting clear reaction mixture was concentrated in vacuo. The crude residue (0.31 g, 98%) was further purified by column chromatography (eluting with 5% Et2O in pentane) to yield compound (3), allicin (0.31 g, 1.9 mmol, 96% yield, purity >95% and up to 99.9%) as a light yellow liquid.
[0067]The above reaction was repeated under varying condition with respect to solvent and temperature. Remaining parameters were kept the same. As seen in table 1 below the reaction is effective under a number of conditions.
TABLE 1Conversion of diallyldisulphide to allicin (3)EntrySolventTemp2 / Yield1Acetone−100° C., 78° C., −50-−20° C.96%2CH2Cl2−100° C., 78° C., −50-−20° C.90%...
example 2
Synthesis of Compound (1), (E,Z)-Ajoene Using Acetone Solvent
[0068]A solution of compound (3), allicin (0.162 g, 1.0 mmol) in a 40% aqueous acetone solution (1.6 mL) was treated with AcOH (0.011 mL, 0.2 mmol). The resulting mixture was heated to 64° C. for 4 h. The cooled reaction mixture was diluted with 50% aqueous methanol (6 mL) and extracted with pentane (5×10 mL). The aqueous fraction was saturated with solid NH4SO4 and the mixture was extracted with CH2Cl2 (5×10 mL). The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The crude residue (0.16 g) was further purified by column chromatography (eluting with 60-80% EtOAc in pentane) to yield compound (1), (E,Z)-ajoene (E:Z ratio=1:4, 0.025 g, 0.1 mmol, 32% yield) as a light yellow liquid.
[0069]The above reaction was repeated using a variety of acids. Remaining parameters were kept the same as above. Exchange of acid had impact on both yields and E:Z ratios of ajoene as seen in table 2 below:
TABLE 2conversi...
example 3
Synthesis of Compound (1), (E,Z)-Ajoene [E:Z=10:1]
[0071]A solution of compound (3), allicin (0.162 g, 1.0 mmol) in a 10% aqueous benzene solution (1.6 mL) was treated with AcOH (0.011 mL, 0.2 mmol). The resulting mixture was heated to 37° C. for 48 h. The cooled reaction mixture was diluted with 50% aqueous methanol (6 mL) and extracted with pentane (5×10 mL). The aqueous fraction was saturated with solid NH4SO4 and the mixture was extracted with CH2Cl2 (5×10 mL). The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The crude residue (0.15 g) was further purified by column chromatography (eluting with 60-80% EtOAc in pentane) to yield compound (1) (E,Z)-ajoene (E:Z ratio 10:1, 0.023 g, 0.1 mmol, 30% yield) as a light yellow liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com