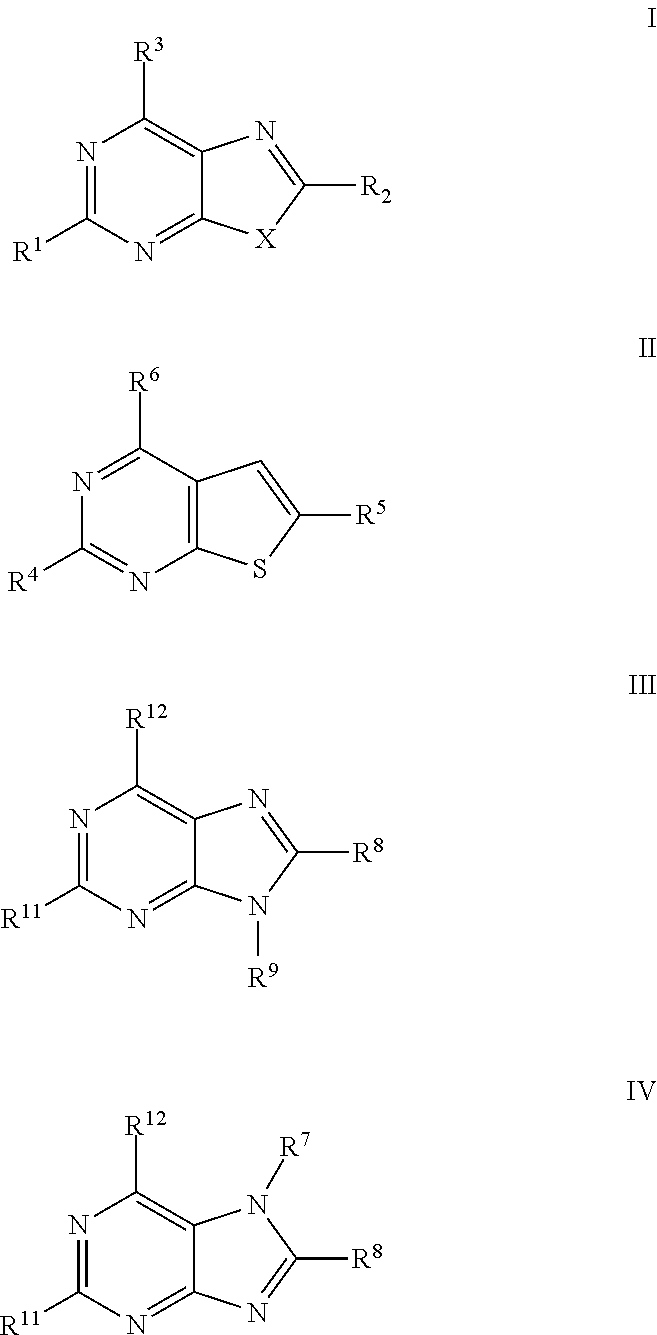
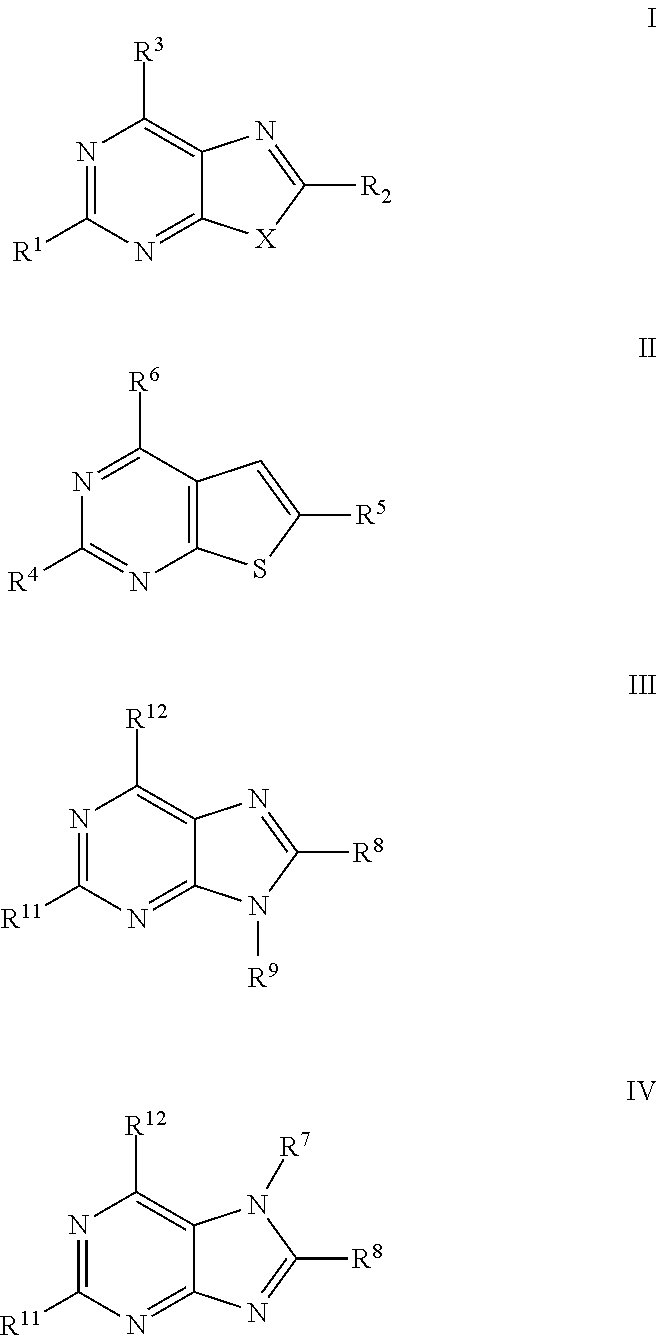
Thiazolopyrimidine Modulators as Immunosuppressive Agents
a technology of thiazolopyrimidine and modulator, which is applied in the field of thiazolo5, 4dpyrimidine, oxazolo5, 4dpyrimidine, thieno2, 3dpyrimidine and purine derivatives, can solve the problems of severe toxic effects on normal cells, limited number of medicinal chemistry papers that describe the synthesis and biological evaluation of oxazolopyrimidines and thiazolopyrimidines, and achieve significant immuno
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of diethyl 2-(4-fluorobenzamido)malonate
[0539]To a solution of diethyl aminomalonate hydrochloride (5.0 g, 23.6 mmol) in pyridine (7.64 ml, 94.5 mmol) and dimethylformamide (60 ml) was added p-fluorobenzoyl chloride (4.19 ml, 35.4 mmol). The reaction mixture was stirred at room temperature for 15 hours. After removing the solvents, the residue was redissolved in dichloromethane, washed with water, brine and dried over Na2SO4. After removing the solvents, the crude residue was purified by flash chromatography on silica (CH2Cl2 / MeOH 30:1) to yield the title compound as a white solid (4.9 g, 70%).
[0540]1H NMR (300 MHz, DMSO, 25° C.): δ=9.37 (d, J=7.5 Hz, 1H, NH), 7.96-8.01 (m, 2H, PhH), 7.33 (t, J=8.8 Hz, 2H, PhH), 5.30 (d, J=7.5 Hz, 1H, CH), 4.12-4.28 (m, 4H, CH2), 1.22 (t, J=7.1 Hz, 6H, CH3) ppm.
[0541]HRMS: calcd for C14H17FNO5 298.1091, found 298.1092.
example 2
Synthesis of diethyl 2-(2-(4-fluorophenyl)acetamido)malonate
[0542]To a solution of 4-fluorophenylacetic acid (4.5 g, 29.3 mmol) and 1-hydroxybenzotriazole (4.35 g, 32.2 mmol) in dichloromethane (140 ml) was added dicyclohexylcarbodiimide (6.65 g, 32.2 mmol). The reaction mixture was stirred at room temperature for 2 hours. The resulting solution was cooled to 00° C., and then a solution of diethyl aminomalonate hydrochloride (6.2 g, 29.3 mmol) in pyridine (4.5 ml, 29.3 mmol) and DMF (10 ml) was added. The temperature was allowed to rise to ambient temperature. After 1 hour, the reaction mixture was concentrated under reduced pressure, and the residue was partitioned between ethyl acetate and a 5% NaHCO3 solution. The organic layer was washed with water, brine and dried over Na2SO4. After removing the solvents, the crude residue was purified by flash chromatography on silica (CH2Cl2 / MeOH 50:1) to yield the title compound as a white solid (8.5 g, 93%).
[0543]1H NMR (300 MHz, CDCl3, 25°...
example 3
Synthesis of dimethyl 2-(3-(4-fluorophenyl)propanamido)malonate
[0545]This compound was synthesized from dimethyl aminomalonate according to the procedure for the preparation of example 2 using 4-fluorophenylpropionic acid. The crude residue was purified by flash chromatography on silica (CH2Cl2 / MeOH 50:1) to yield the title compound as a white solid (53%).
[0546]1H NMR (300 MHz, CDCl3, 25° C.): δ=7.13-7.18 (m, 2H, PhH), 6.96 (t, J=8.7 Hz, 2H, PhH), 6.50 (d, J=6.7 Hz, 1H, NH), 5.19 (d, J=6.7 Hz, 1H, CH), 3.80 (s, 6H, CH3), 2.95 (t, J=7.4 Hz, 2H, CH2), 2.58 (t, J=7.4 Hz, 2H, CH2) ppm.
[0547]HRMS: calcd for C14H17FNO5 298.10908, found 298.10827.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com