Low acidic species compositions and methods for producing and using the same using displacement chromatography
a technology of displacement chromatography and compositions, applied in the direction of antibody medical ingredients, tumor necrosis factor, peptides, etc., can solve the problems of charge variant reduction, achieve the effects of improving biological properties, improving therapeutic efficacy, and increasing cartilage tissue penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Displacement Chromatography Performances of Expell SP1™ for Adalimumab on Poros XS Resin
[0431]Expell SP1™ is a low molecular weight quaternary ammonium salt that exhibited pronounced displacement effect for Adalimumab on Poros XS resin under selected sets of operating conditions. The feed material used for this set of experiments contained about 20-25% total AR, of which 2-5% was AR1 and 18-20% AR2. The results for this system are shown in the following sections.
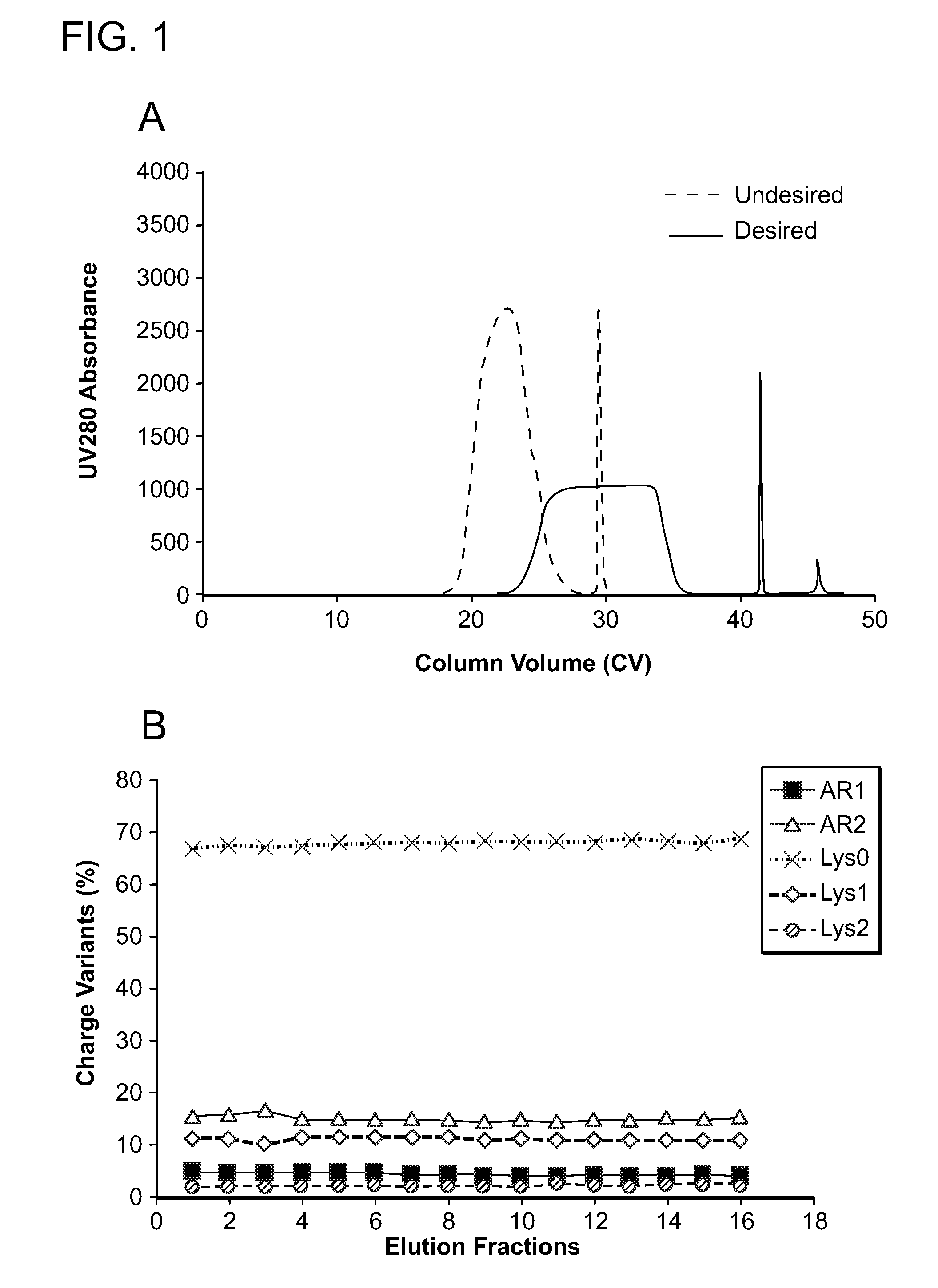
[0432]A representative, desired displacement chromatographic profile is shown in FIG. 1a (solid line). In this experiment, the column was equilibrated with a pH 7 Tris / acetate buffer (6.4 mS / cm), loaded with a pre-adjusted protein A eluate feed (pH 7.5, 6.3 mS / cm, ˜3.4 g / L) to ˜40 g / L resin loading level, followed by EQ buffer wash and then displacement process using 1 mM Expell SP1™ in the pH 7 EQ buffer. The extended, square shape UV280 “elution” profile indicated establishing a proper displacement train and thus a degree ...
example 2
Displacement Chromatography Performance of Protamine Sulfate for Adalimumab on Poros XS Resin
[0443]Protamine sulfate, a cationic peptide with molecule weight ˜5.1 kD, was also evaluated as a cation exchange displacer for Adalimumab on Poros XS resin under various operating conditions. The feed material used for this set of experiments contained about 17-24% total AR, of which 3-6% was AR1 and 14-19% AR2. The results for this system are illustrated in the following sections.
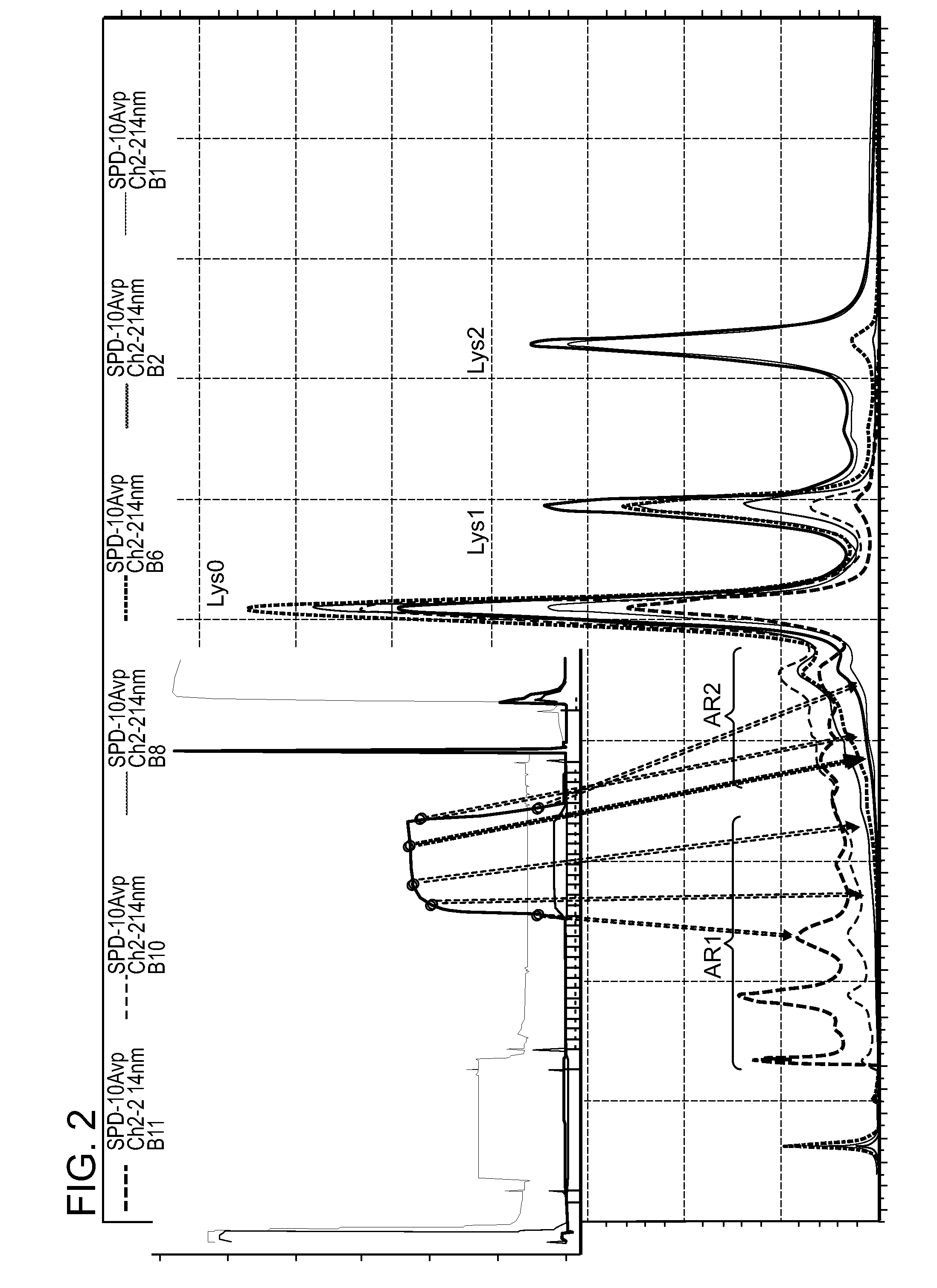
[0444]FIG. 10 shows the distribution of charge variant species in sample fractions collected from a well established displacement process induced by protamine sulfate. In this experiment, the column was equilibrated with a pH 7.5 Tris / acetate buffer (5.6 mS / cm), loaded with a pre-adjusted protein A eluate feed (pH 7.5, 5.4 mS / cm, 5.2 g / L) to 39 g / L resin loading level, followed by a brief EQ buffer wash and then displacement process using 0.5 mM protamine sulfate dissolved in the pH 7.5 EQ buffer. Similar to the E...
example 3
Displacement Chromatography Performance of Expell SP1™ for mAb X on Poros XS Resin
[0450]The displacement separation performance of Expell SP1™ was assessed for mAb X on the Poros XS resin. A purified mAb X drug substance was used in this study, which contained about 16-17% acidic species and 12-14% basic species.
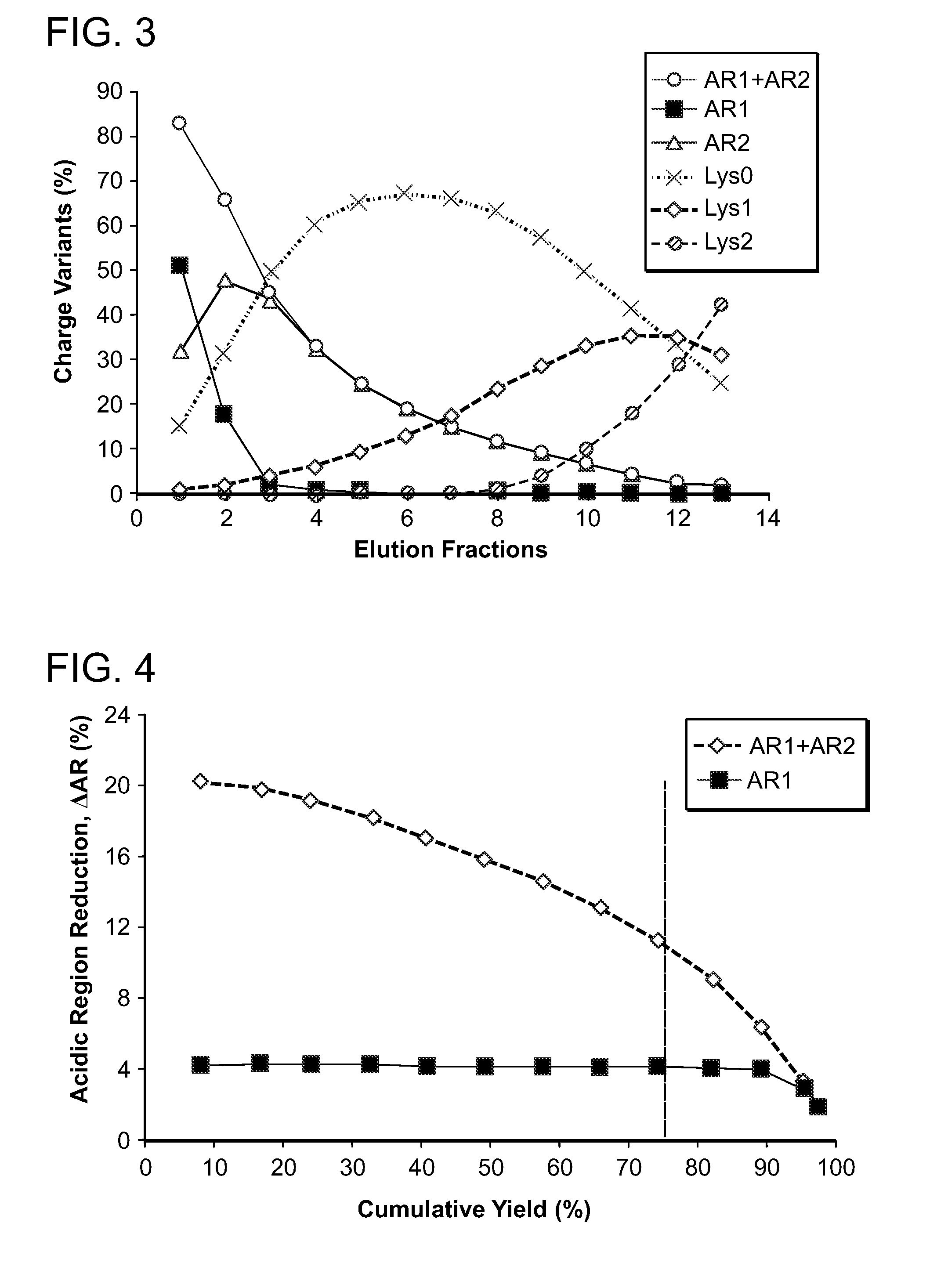
[0451]A representative set of mAb X charge variant separation profiles are shown in FIGS. 17 and 18. In this experiment, the Poros XS column was loaded with 40 g / L of mAb X at pH 6, 6 mS / cm Tris / Acetate binding condition, and was displaced using 1 mM Expell SP1™ in a pH 6, ˜6 mS / cm buffer. The specific conditions are detailed in Table 1. Pronounced enrichment and separation of acidic, main and basic species were achieved, with AR % reduced by 9.4% at 76% yield.
[0452]The effect of Expell SP1™ concentration on AR reduction for mAb X was measured in the pH 6 Tris / Acetate buffer. As shown in FIG. 19, increasing the Expell SP1™ concentration from 0.5 to 2 mM decreased the ΔAR % f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com