Pharmaceutical formulations comprising neurotrophin mimetics
a technology of neurotrophin and formulation, applied in the field of neurodegenerative and other disorders, can solve the problems of increasing the chance of adverse effects and ligands not engaging, and achieve the effect of improving the chances of adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Computational Modeling, Pharmacophore Generation, Virtual and Functional Screening
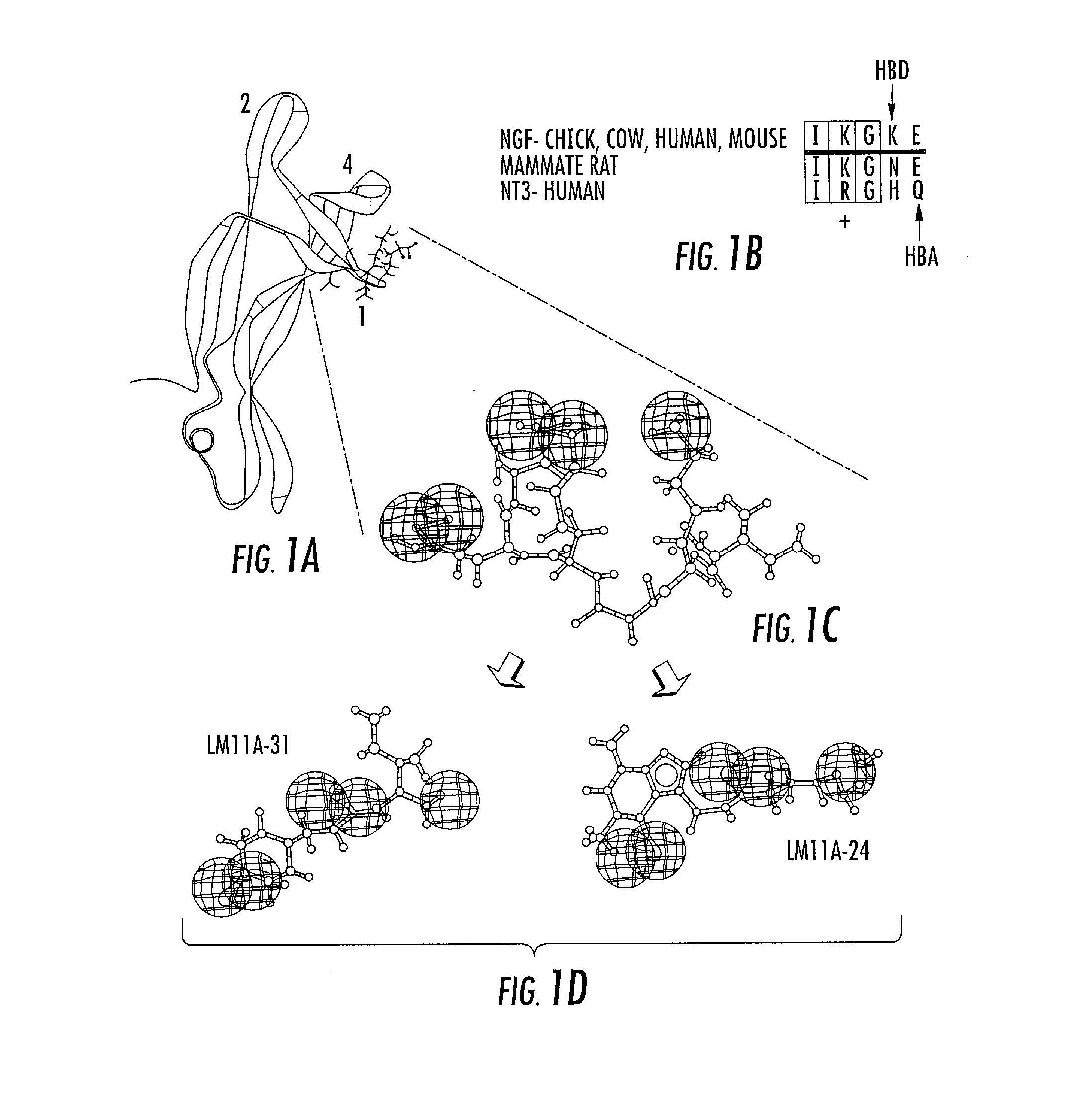
[0229]In order to generate a productive pharmacophore emulating a loop structure likely to interact with a receptor, it was hypothesized that (1) the degrees of freedom of the ligand peptide structure are restricted by its residence in the protein, and (2) there is little “induced fit” involving changes in loop structure at the targeted receptor subsite, or it is accommodated by flexibility of the small molecule ligand. When both of these conditions apply, they allow an interacting / activating small molecule conformation that interacts with the receptor in a manner similar to that of the native ligand.
[0230]Computational studies of active dimeric cyclic peptides mimicking NGF β-hairpin loops suggest that energetic and structural constraints would disallow simultaneous β-hairpin folding of both peptide subunits, implying that the peptides act in a monomeric fashion. Additionally, based on early virtual s...
example 2
Compounds Promote Hippocampal Neuron Survival
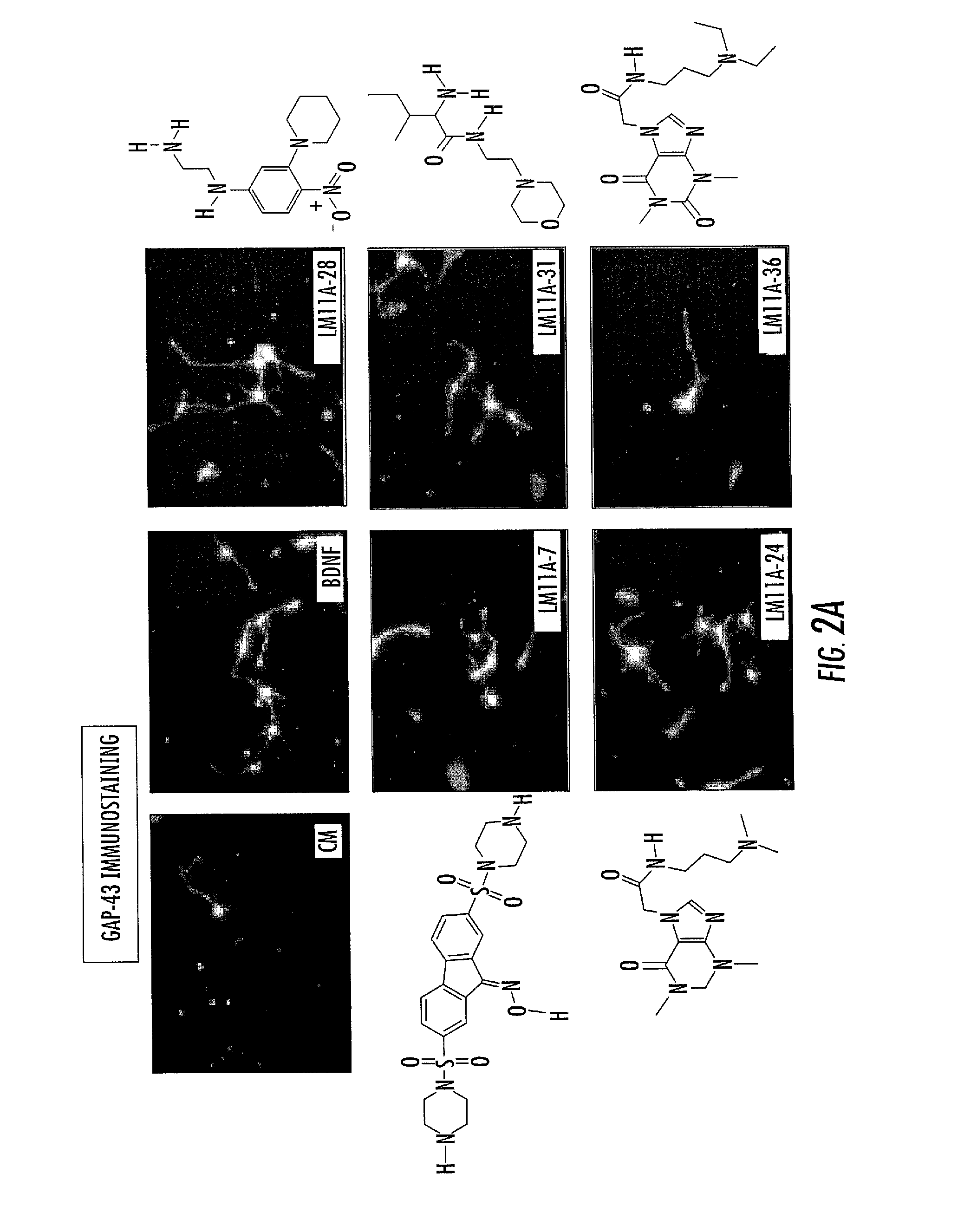
[0235]High-throughput virtual screening based on neurotrophin loop 1 models and small-scale in vitro bioassays were used to identify chemically diverse compounds with potent neurotrophic activity (FIG. 1). Approximately 800,000 compounds were screened in silico to produce a high yield of 4 positives out of 23 compounds submitted to in vitro screening (17%).
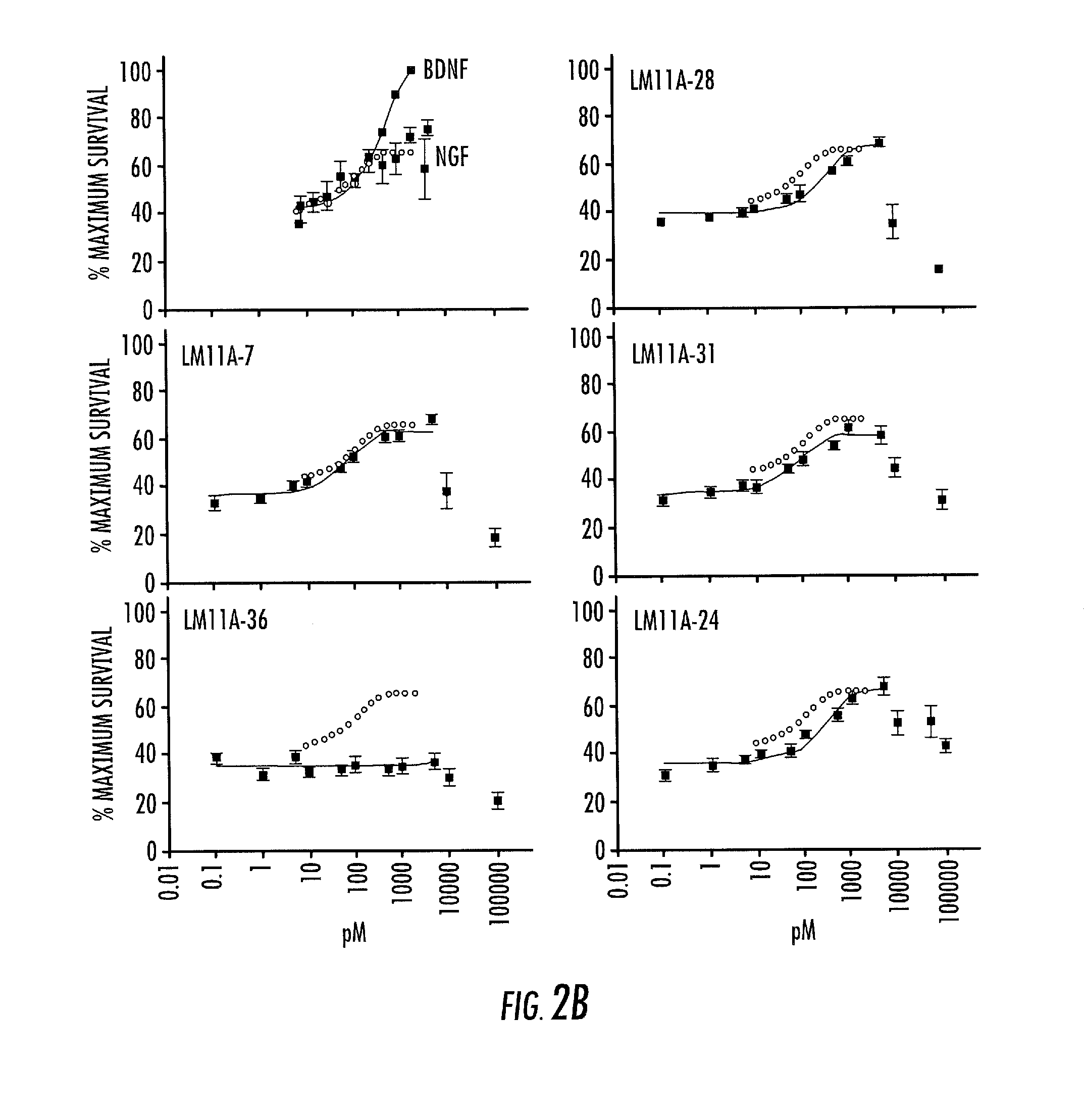
[0236]In order to understand the mechanisms of action of the selected compounds and test the conjecture that they work via the targeted receptor, p75NTR the dose-dependent relationships of the survival-promoting activities of the p75-binding compounds compared to NGF and BDNF using embryonic hippocampal neurons in culture conditions in which NGF promotes neural survival were examined. In the cultures, neurotrophic activity was mediated by BDNF principally through TrkB and p75NTR and by NGF primarily through p75NTR, as they express little TrkA (Brann, A. B., et al. (1999) J Neurosci 19, ...
example 3
Compounds Interact With and Work Through p75NTR Receptors, Not Trk Receptors
[0241]In order to assess the interactions of the p75-binding compounds with neurotrophin receptors, the effects of increasing concentrations of compounds on NGF binding to the recombinant chimeric proteins p75NTRNTR-Fc and TrkA-Fc were examined. In these experiments, Compound 4 (FIG. 3a) and Compound 3 (FIG. 3b), but not Compound 5 (FIG. 3c), shifted the NGF / p75NTR-FC biding curve significantly to the right. The inhibition of NGF binding caused by each active compound was reversed with increasing NGF concentration, consistent with a mechanism that is, at least in part, competitive in nature. When the data was fit to the Gaddum-Schild equation that describes ligand binding in the presence of an inhibitor (Motulsky, H.J., and Christopoulos, A. (2003) A Practical Guide to Curve Fitting, 2nd edn. (San Diego, Calif., GraphPad Software, Inc.)), the resulting Schild coefficients were significantly less than 1.0 for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com