Method and System for Controlling Electrical Conditions of Tissue II
a technology of electrical conditions and tissue, applied in the field of tissue electrical conditions control, can solve the problems of difficult task, presenting a significant obstacle to isolating the cap of interest, and the precise mechanisms involved are poorly understood, so as to reduce the complexity of the measurement signal chain, reduce the interaction, and reduce the effect of artefa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
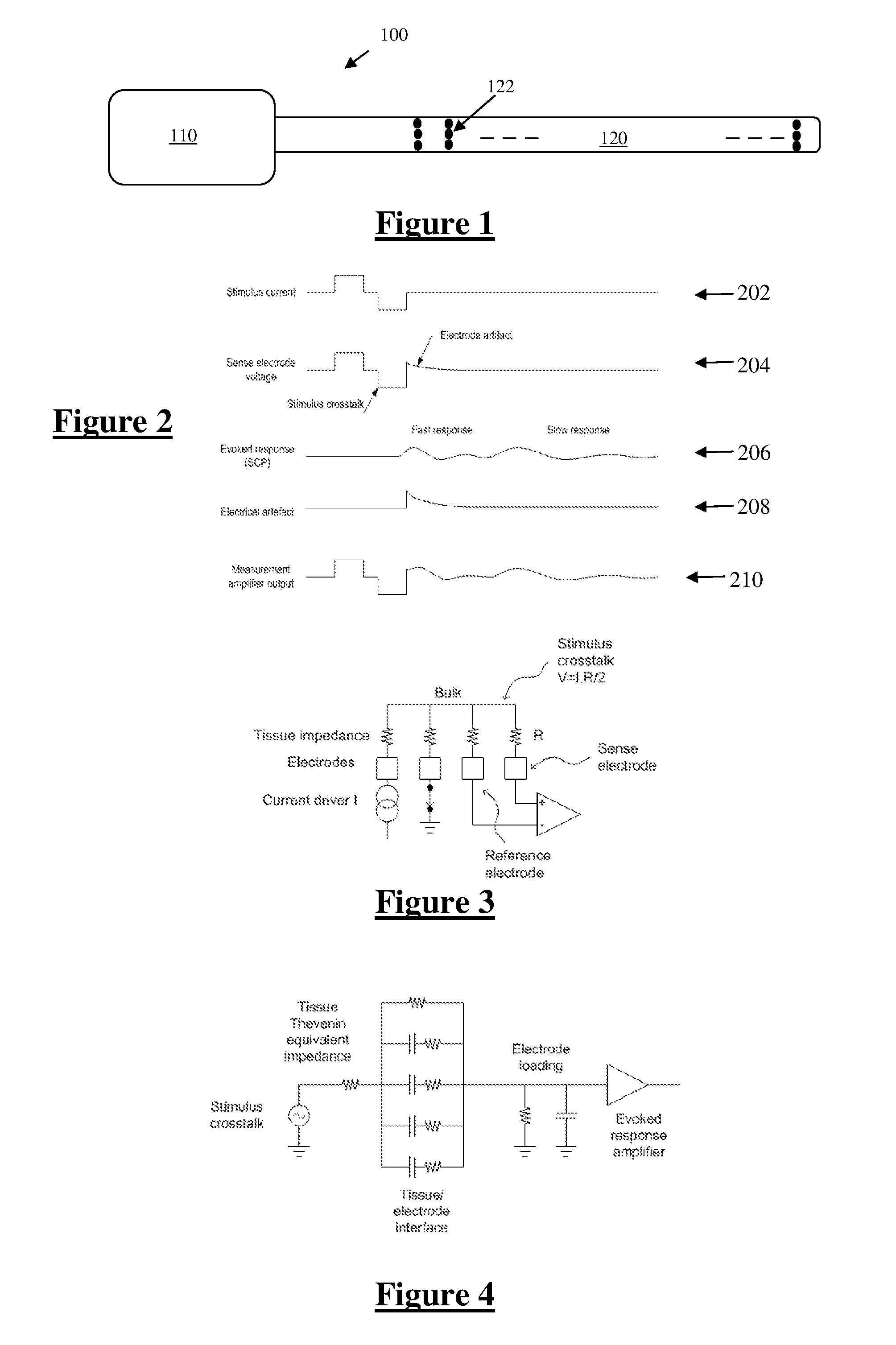
[0050]FIG. 1 illustrates an implantable device 100 suitable for implementing the present invention. Device 100 comprises an implanted control unit 110, which controls application of a sequence of neural stimuli. In this embodiment the unit 110 is also configured to control a measurement process for obtaining a measurement of a neural response evoked by a single stimulus delivered by one or more of the electrodes 122. Device 100 further comprises an electrode array 120 consisting of a three by eight array of electrodes 122, each of which may be selectively used as the stimulus electrode, sense electrode, compensation electrode or sense electrode.
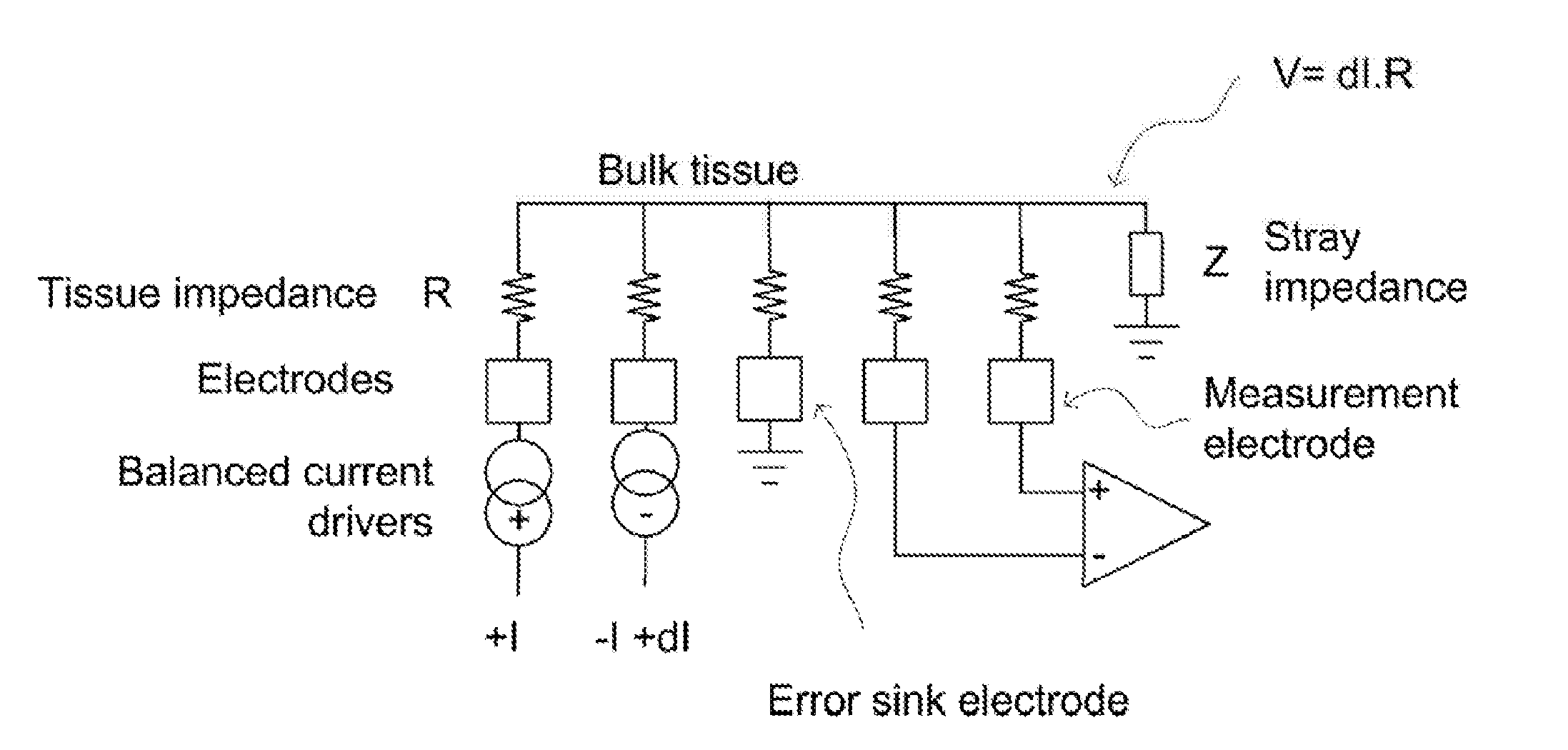
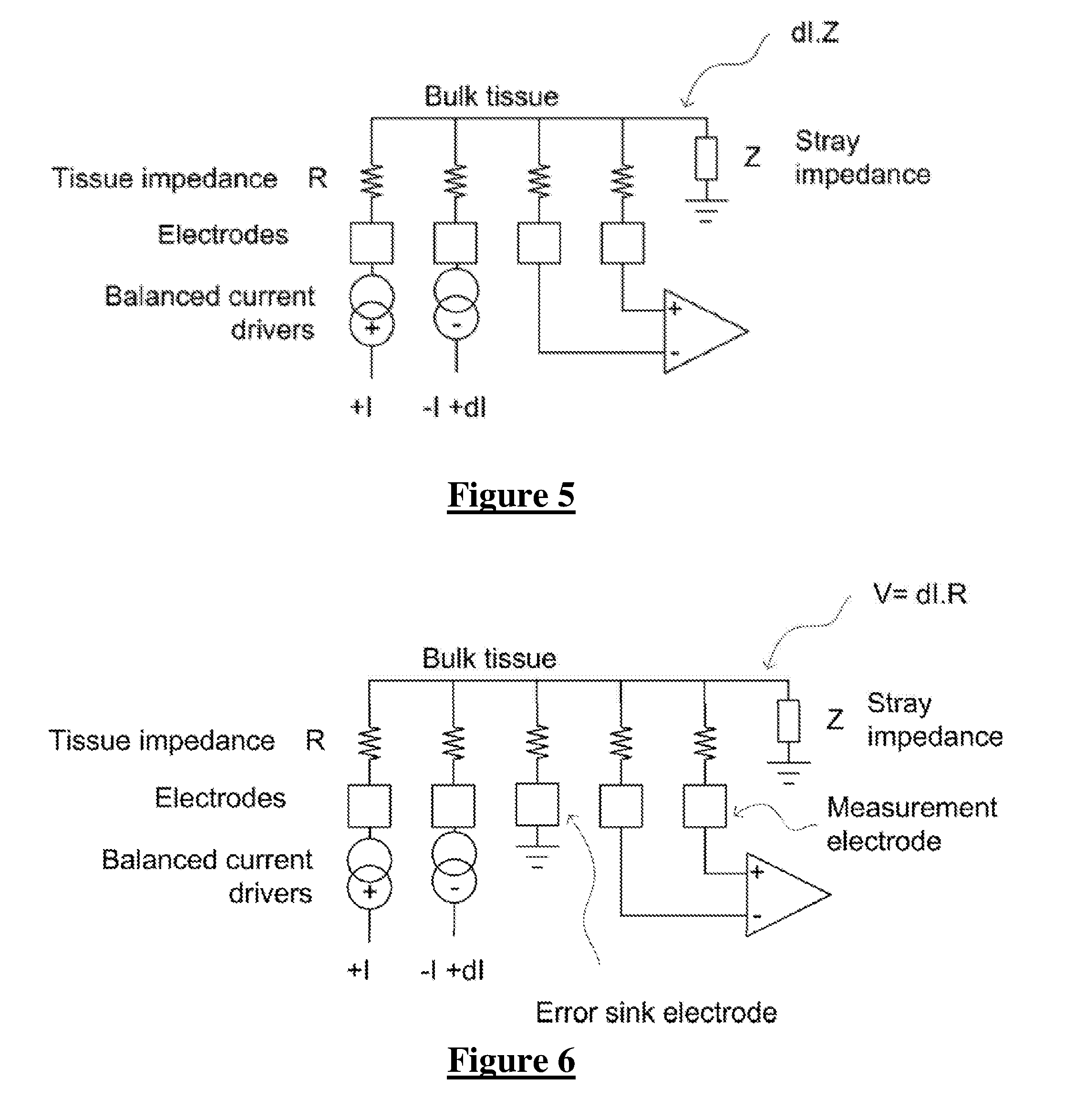
[0051]FIG. 2 shows the currents and voltages that contribute to spinal cord potential (SCP) measurements in a typical system of the type shown in FIG. 3. These signals include the stimulus current 202 applied by two stimulus electrodes, which is a charge-balanced biphasic pulse to avoid net charge transfer to or from the tissue and to provide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com