Fluorogenic dendrimer reporters and related methods of use
a technology of dendrimer reporter and fluorescence, which is applied in the field of fluorogenic dendrimer reporter, can solve the problems of limited application of small molecule fluorescent reporter to monitor biological systems, and achieve the effects of reducing background noise, strong fluorescence enhancement, and efficient monitoring of biological processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0133]Previous experiments involving dendrimer related technologies are located in U.S. Pat. Nos. 6,471,968, 7,078,461; U.S. patent application Ser. Nos. 09 / 940,243, 10 / 431,682, 11,503,742, 11,661,465, 11 / 523,509, 12 / 403,179, 12 / 106,876, 11 / 827,637, 10 / 039,393, 10 / 254,126, 09 / 867,924, 12 / 570,977, and 12 / 645,081; U.S. Provisional Patent Application Ser. Nos. 61 / 256,699, 61 / 226,993, 61 / 140,480, 61 / 091,608, 61 / 097,780, 61 / 101,461, 61 / 251,244, 60 / 604,321, 60 / 690,652, 60 / 707,991, 60 / 208,728, 60 / 718,448, 61 / 035,949, 60 / 830,237, 61 / 736,225, and 60 / 925,181; and International Patent Application Nos. PCT / US2010 / 051835, PCT / US2010 / 050893; PCT / US2010 / 042556, PCT / US2001 / 015204, PCT / US2005 / 030278, PCT / US2009 / 069257, PCT / US2009 / 036992, PCT / US2009 / 059071, PCT / US2007 / 015976, and PCT / US2008 / 061023.
example 2
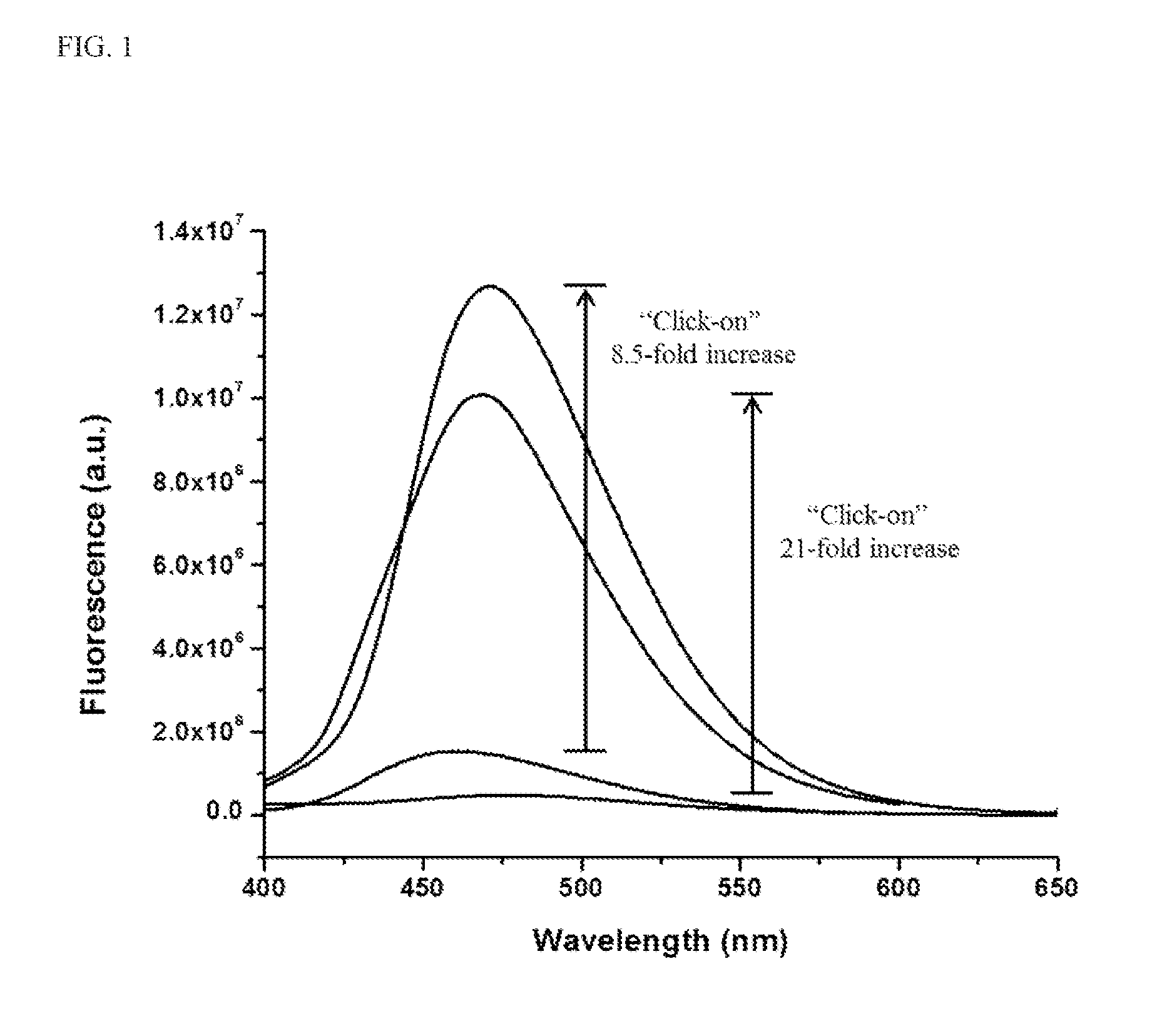
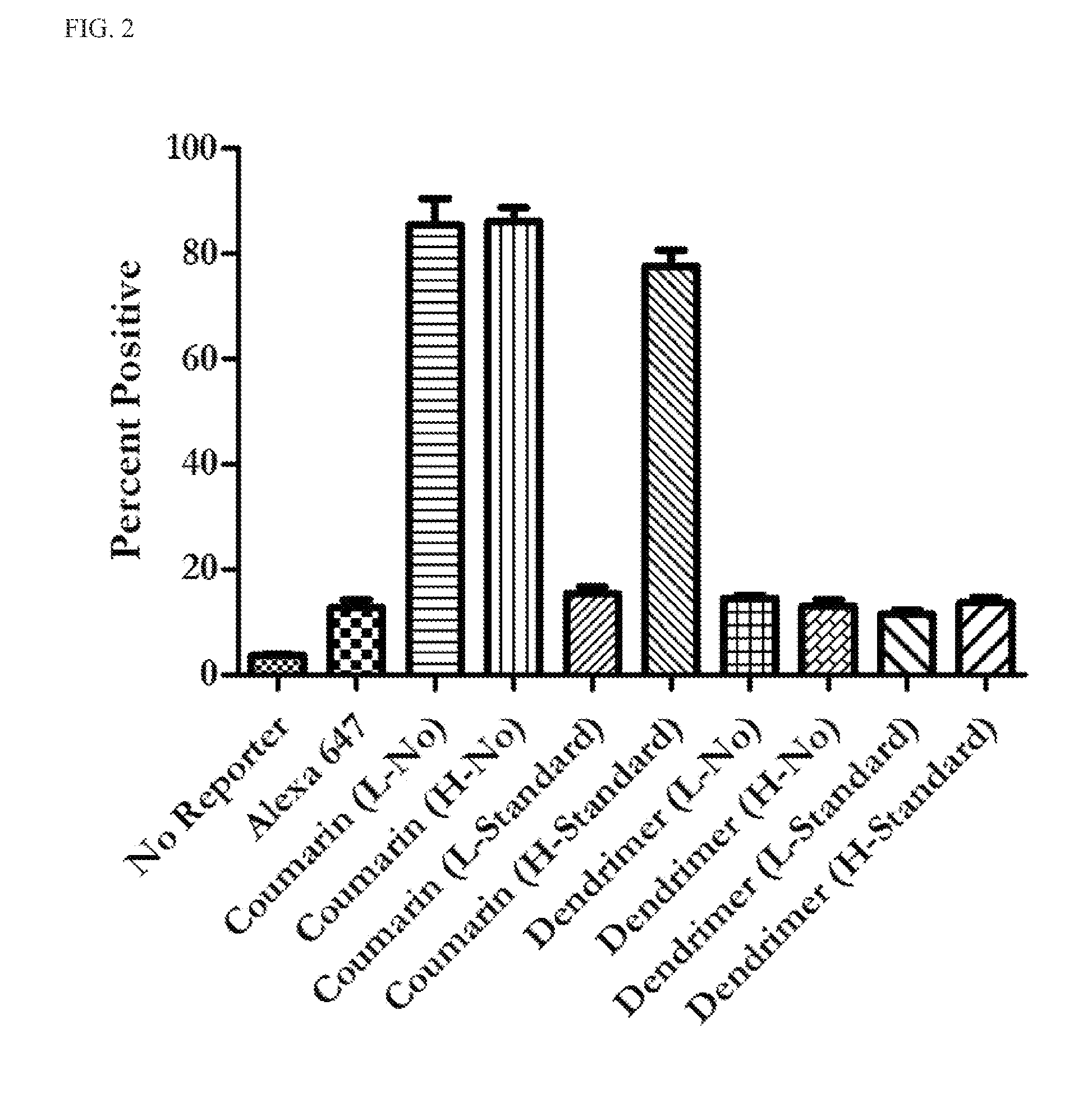
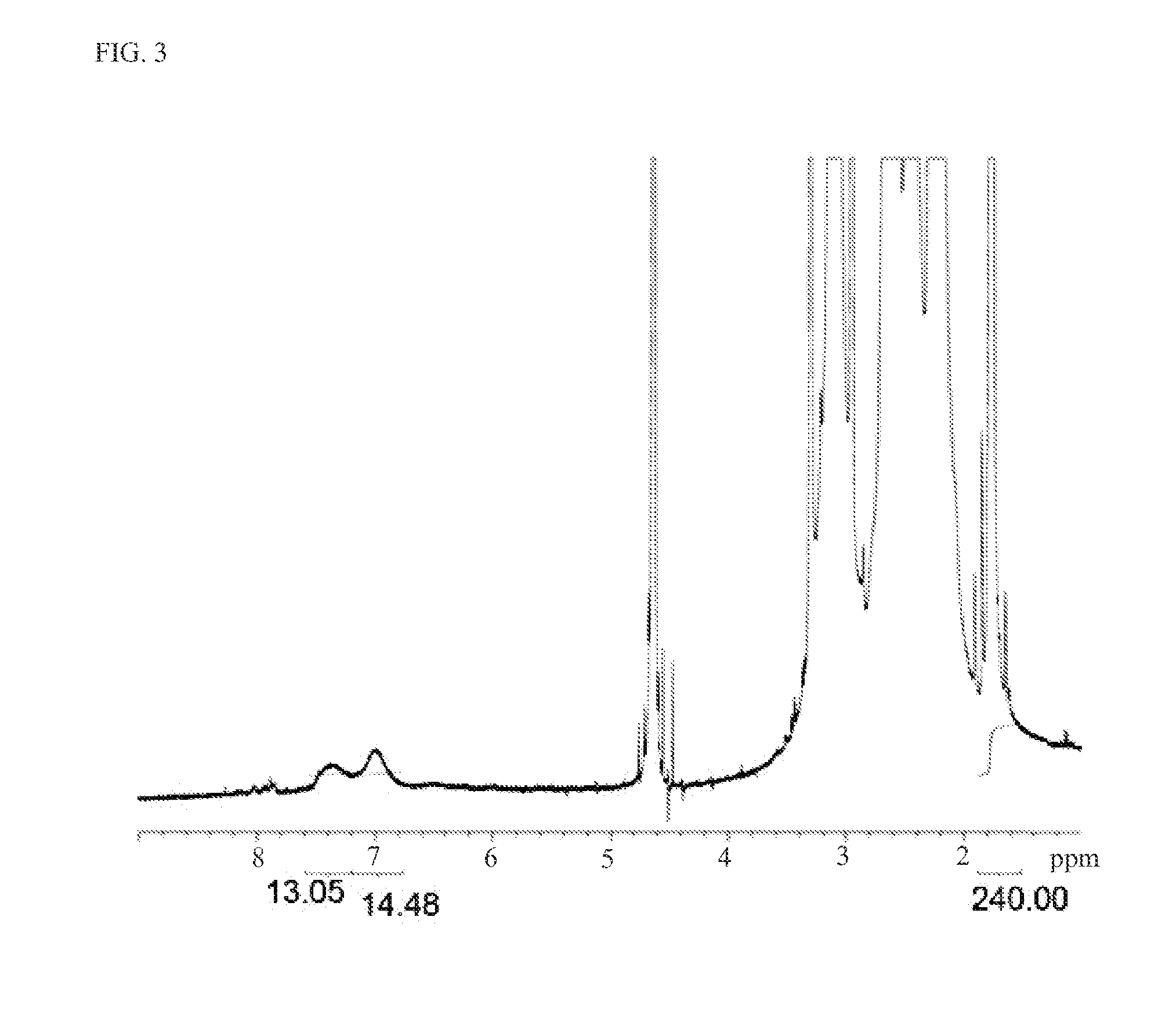
[0134]This example describes fluorogenic dendrimer reporters of the present invention. The fluorogenic dendrimer reporters of the present invention are composed of a partially acetylated G5 PAMAM dendrimer (see, e.g., Majoros, I. J.; Thomas, T. P.; Mehta, C. B.; Baker, J. R. J Med Chem 2005, 48, 5892), a fluorogenic 3-azido-7-hydroxy coumarin dye, and a triazine ring linker (Scheme 1). The synthetic strategy is comprised of three parts: (1) conjugation of 3-azido-7-hydroxy coumarin to 2,4,6-trichloro-1,3,5-triazine at 0° C., triazine-coumarin 1; (2) G5 dendrimer was partially acetylated (70%) with acetic anhydride, G5-NHAc-NH2 2; (3) conjugation of triazine-coumarin to the partially acetylated G5 dendrimer at room temperature, G5-Coumarin 3. The ease of displacement of the chlorine atoms in 2,4,6-trichloro-1,3,5-triazine by various nucleophiles in a controlled manner makes this reagent useful to serve as a cross-linker between G5 dendrimer and 3-azido-7-hydroxy coumarin. After react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com