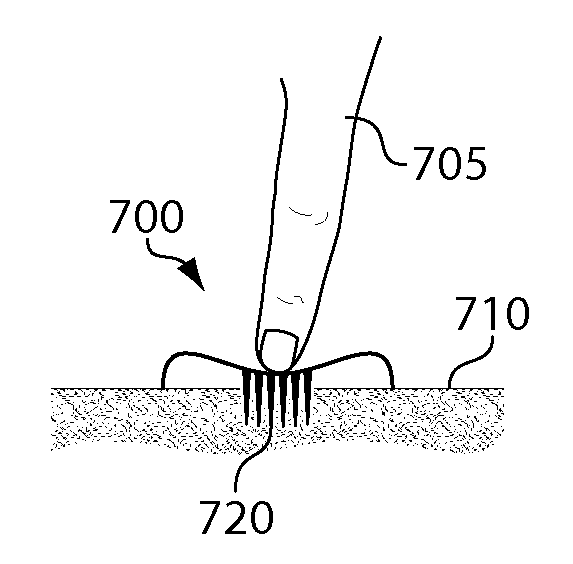
Sampling devices and methods involving relatively little pain
a sampling device and relatively little technology, applied in the field of relatively little pain sampling device, can solve the problems of complex procedures, inability to perform non-medical settings, and require sophisticated training of practitioners, and achieve the effects of high acceleration, high energy, and speed of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
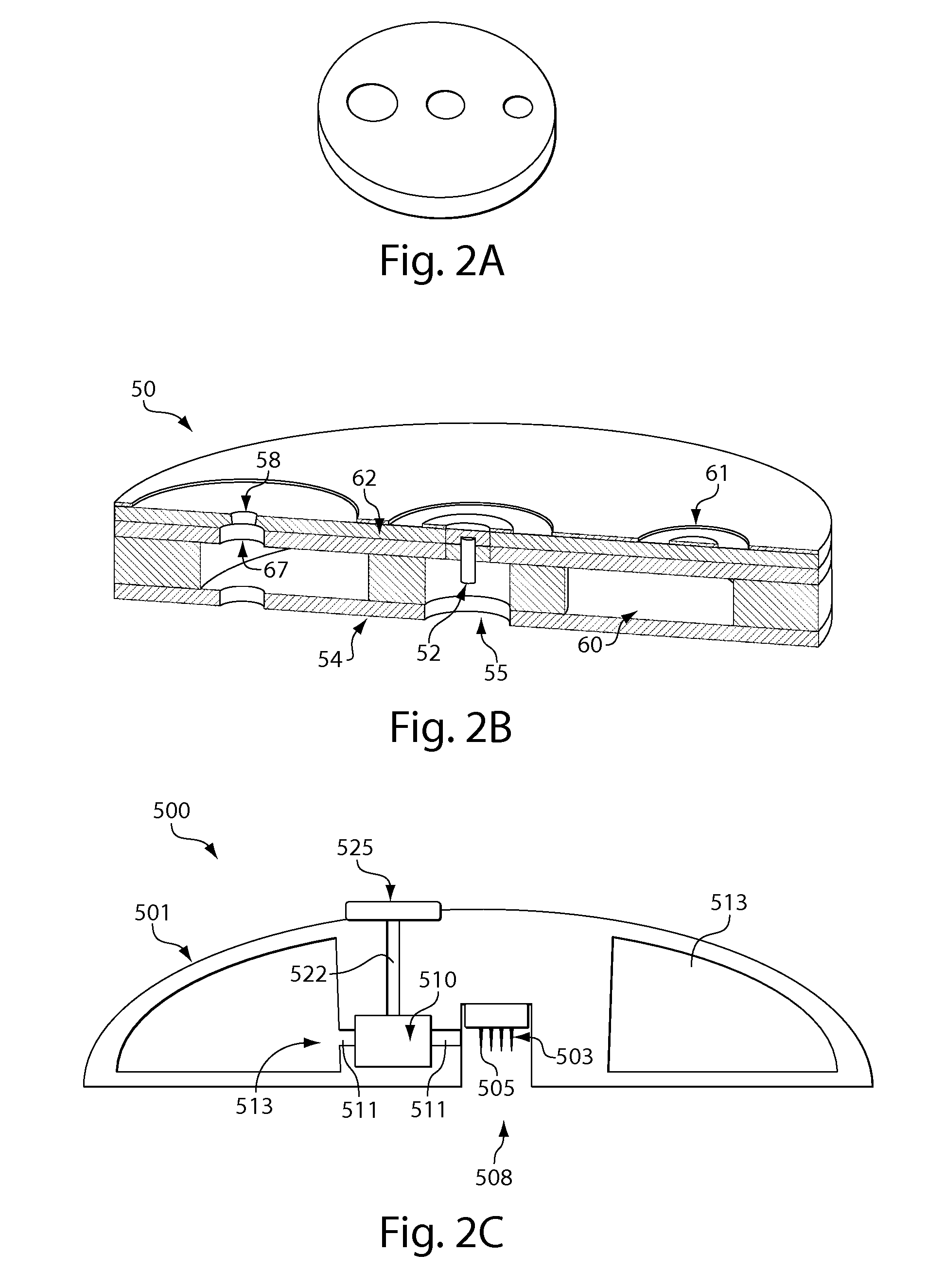
[0254]In this example, various devices as discussed above were applied to subjects and the degree of pain evaluated using the Wong-Baker FACES Pain Scale, as compared to a commercially available device. The devices included various microneedles in fluidic communication with a reservoir for storing blood received from the device. A prepackaged vacuum on the device was used to extract the blood, and the microneedles were injected by the device into the skin using a “snap dome” such as is shown in FIG. 11.
[0255]Four such devices were tested: the first device include 4 microneedles, each 750 micrometers long (“4×750”); the second device included 8 microneedles, each 750 micrometers long (“8×750”); the third device included 16 microneedles, each 750 micrometers long (“16×750”); and the fourth device included 4 microneedles, each 1000 micrometers long (“4×1000”). All of the microneedles were solid. The fifth control device tested was the commercially available Accu-Check Multiclix device ...
example 2
[0257]This example illustrates a device for inserting a microneedle array into the skin of a subject. FIG. 15A shows a device 800 including a substance transfer component (e.g., including a microneedle array 833), a deployment actuator (e.g., snap dome 832), an activator (e.g., activation button 831), a retraction actuator (e.g., silicone foam 835), and structural components constructed from multiple layers of polycarbonate bonded together using a double-sided adhesive, such as 3M 1509 or 3M 1513 tape. The microneedle arrays can be bonded to laminated post 837 on the underside of a snap dome. Structural components 838, as well as post 837, are formed from polycarbonate and 3M 1509 or 3M 1513 adhesive. The arrays may range in needle number (4 to 28 needles), needle length (350 to 1000 micrometers), and / or arrangement (square, rectangular, and circular arrays), with array footprints of less than 3 mm in diameter, where the “footprint” is the area of the base to which the needles are a...
example 3
[0259]This example illustrates a device for withdrawing blood using vacuum. This device is shown in FIG. 15B. This device includes a vacuum chamber comprising layers of polycarbonate, polyethylene terephthalate glycol (PETG), and silicone bonded together using a double-sided adhesive, such as 3M 1509 or 3M 1513 tape. The chamber is approximately 2.7 cm in diameter and 0.6 cm high, with cup opening 858 (substance transfer component) in the base that ranges from 3 to 7 mm in diameter. The vacuum chamber may be attached to the skin of a subject over the microneedle insertion site using adhesive 857, such as 3M 1509 or Katecho 10G hydrogel. A vacuum source (i.e., vacuum pump, syringe, vacuum reservoir, etc.) can be connected to the chamber using hypodermic needle 859 inserted through silicone layer 852, and vacuum (i.e., 30 to 70 kPa) may be applied to the site for a fixed period of time (i.e., 10 s to 10 min). The application of vacuum causes blood to flow from the skin punctures into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com