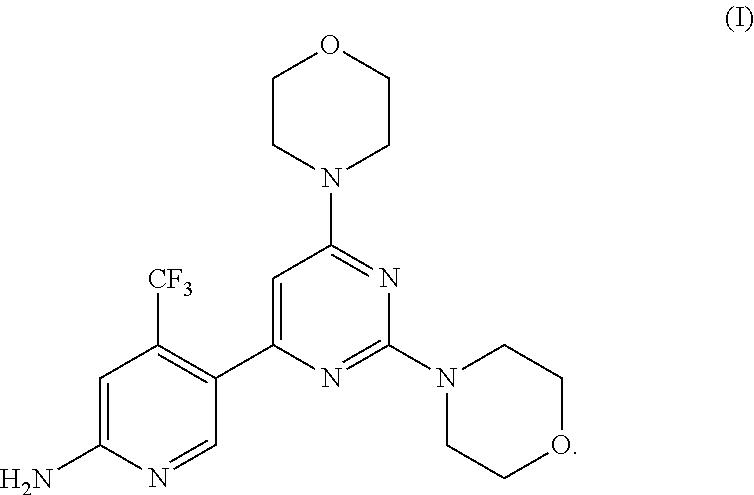
Combination of a PI3 Kinase Inhibitor with Pacitaxel for Use in the Treatment or Prevention of a Cancer of the Head and Neck
a technology of pacitaxel and kinase inhibitor, which is applied in the field of combination of pi3 kinase inhibitor and pacitaxel for use in the treatment or prevention of head and neck cancer, can solve the problems of poor recurrence rate, poor progress, and ineffective palliative treatment of recurrent/metastatic hnscc, so as to reduce side effects, prolong the response, and delay the progression or inhibit the symptoms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sensitivity of Head and Neck Cancer Cell Lines to Compound A and to a Combination of Paclitaxel and Compound A
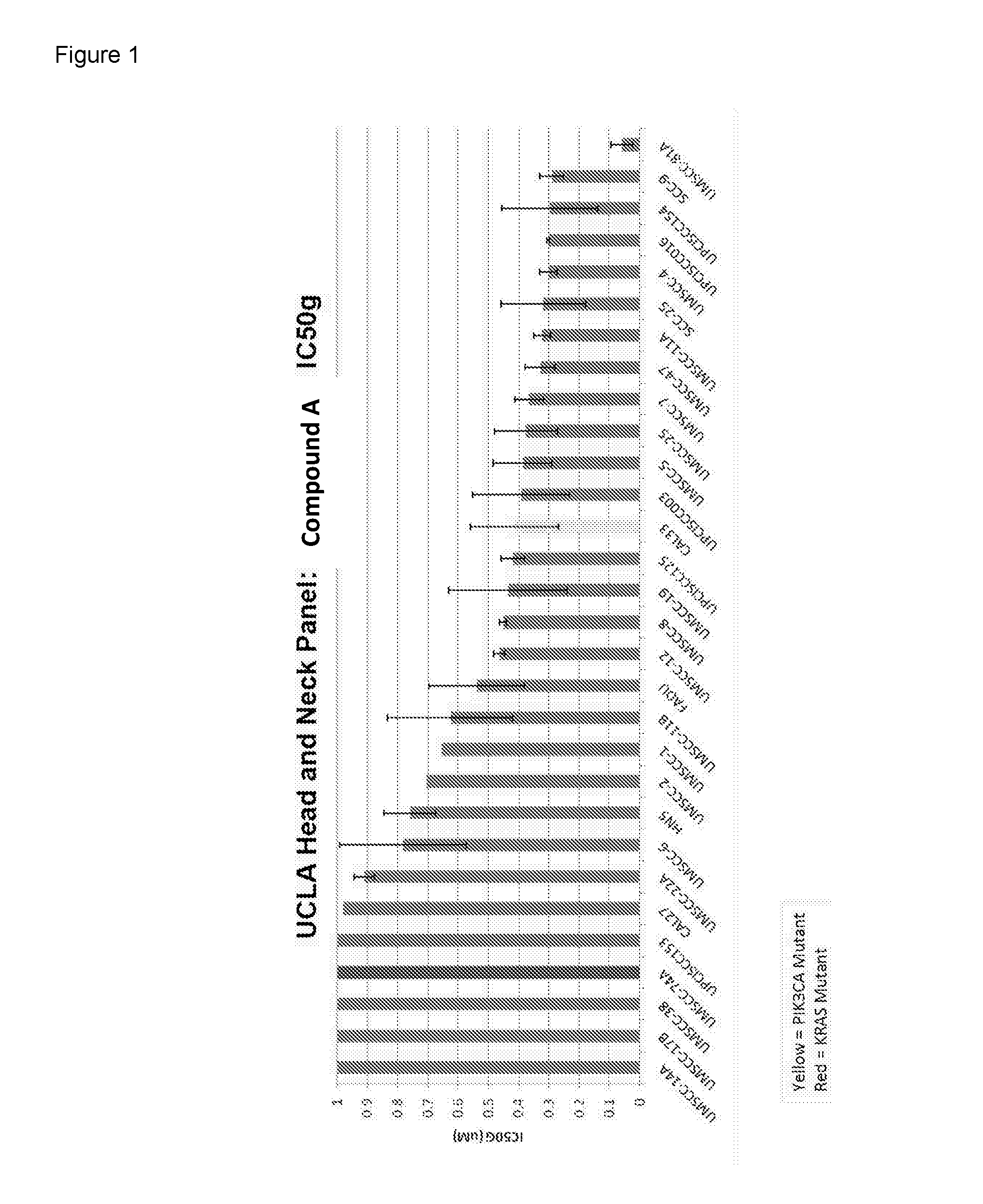
[0103]As shown in FIG. 1, two panels were independently tested for sensitivity to Compound A. The majority of the cell lines display an IC50 below 1 μM in line with clinically relevant concentrations (concentration delivered to patients treated at 100 mg daily is expected to be around 1 μM). (Red=KRAS mutant=UMSCC-74A. Yellow=PI3KCA mutant=CAL33).
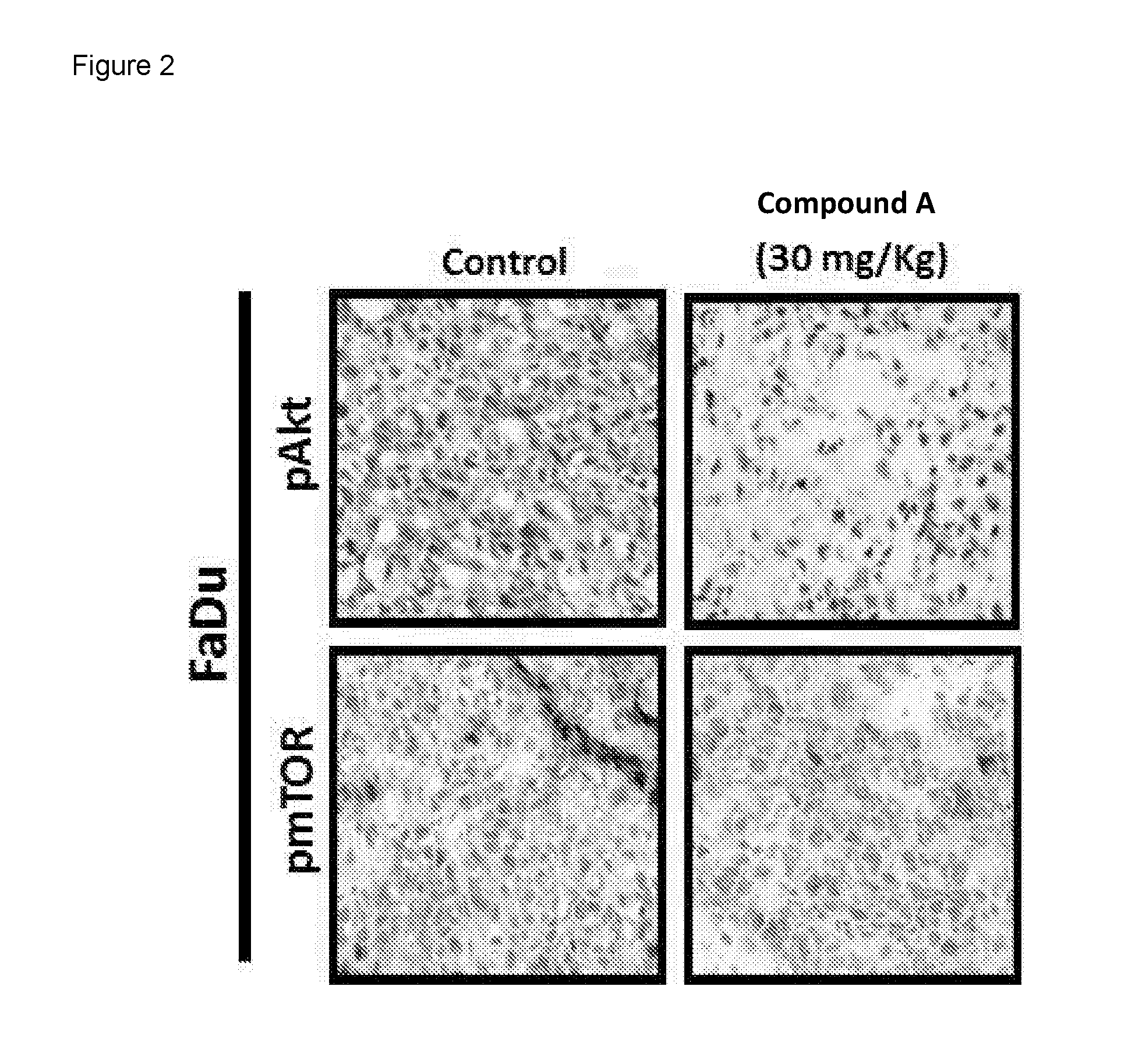
[0104]As shown in FIG. 2, treatment of FaDu xenograft (Hypopharynx squamous cell carcinoma) with Compound A at 30 mg daily (equivalent to 100 mg daily in patients) shows inhibition of pAKT in tumor tissue confirming down-regulation of the PI3K pathway upon treatment. Treatment with Paclitaxel and Compound A in head and neck cancer cell lines displays combination effect with potential for synergy in some cases.
[0105]Cells were plated in 24-well plates at a density of 5×104 to 1×105 cells per well and grown in DMEM with 10% FBS and 1%...
example 2
[0106]A clinical study using (a) a phosphatidylinositol 3-kinase inhibitor COMPOUND A or its hydrochloride salt, in combination with (b) paclitaxel for treatment of patients with recurrent or metastatic HNSCC cancer that has progressed after prior platinum based treatment regimen.
[0107]A multi-center, randomized, double-blind, placebo-controlled phase II trial of the combination comprising (a) COMPOUND A or its hydrochloride salt and (b) paclitaxel is conducted in patients with recurrent or metastatic HNSCC cancer that has progressed after prior platinum based treatment regimen. Patients with histologically / cytologically-confirmed HNSCC, recurrent or metastatic disease progressing after prior platinum-based first-line treatment will be randomized in a 1:1 ratio to 2 different clinical group arms to receive in a blinded manner one of two treatments: (a) COMPOUND A or its hydrochloride salt in combination with paclitaxel, or (b) placebo in combination with paclitaxel. Ap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com