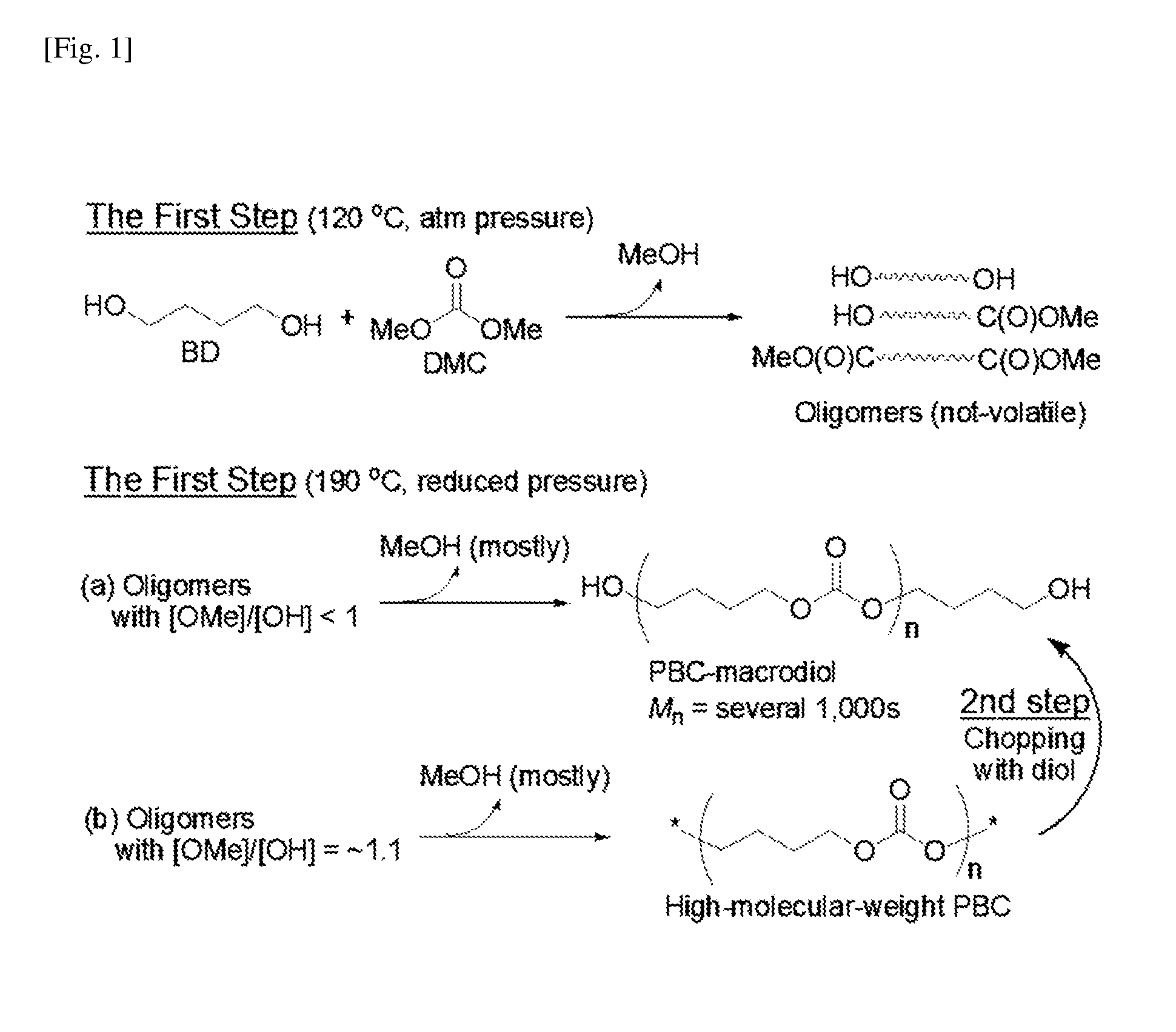
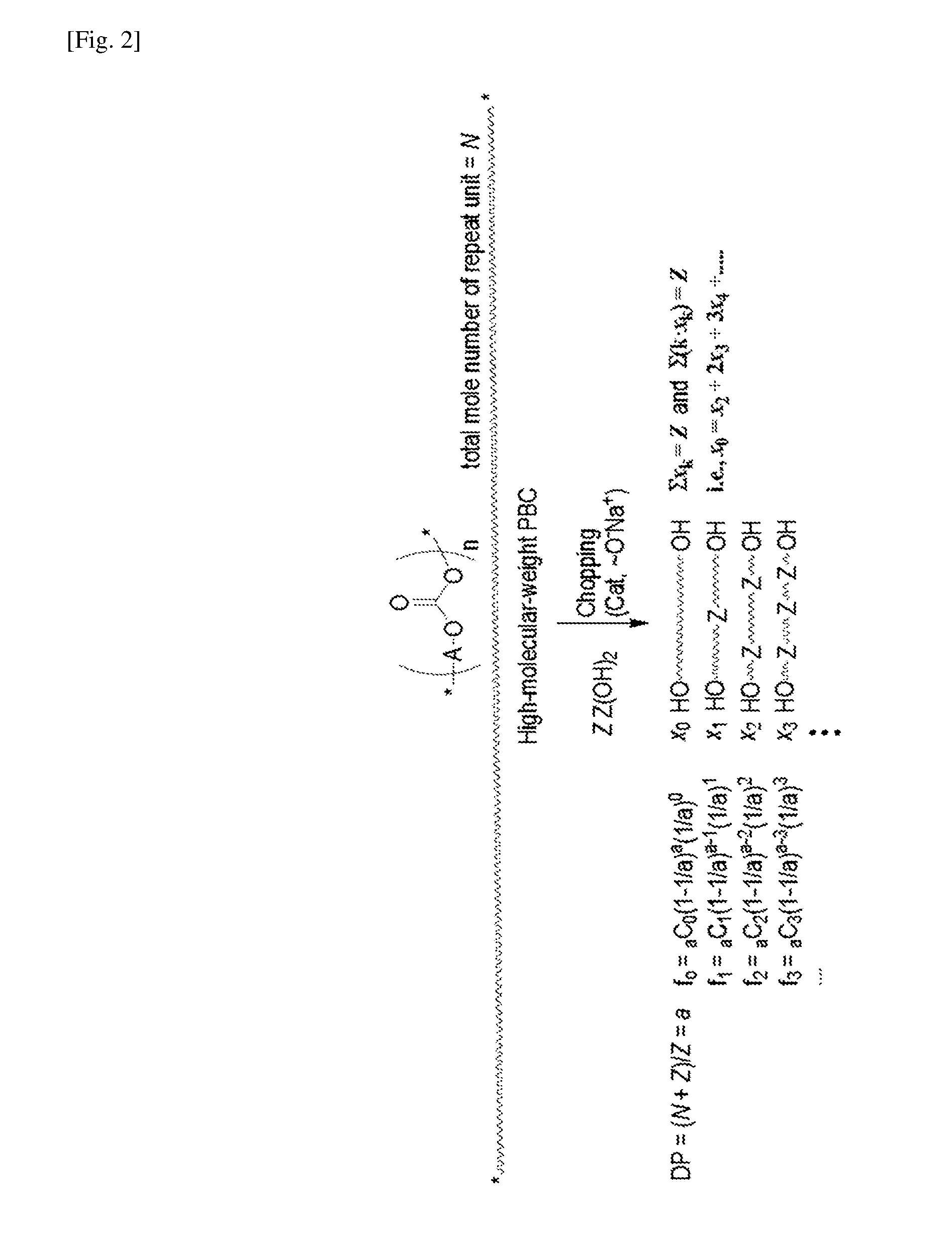
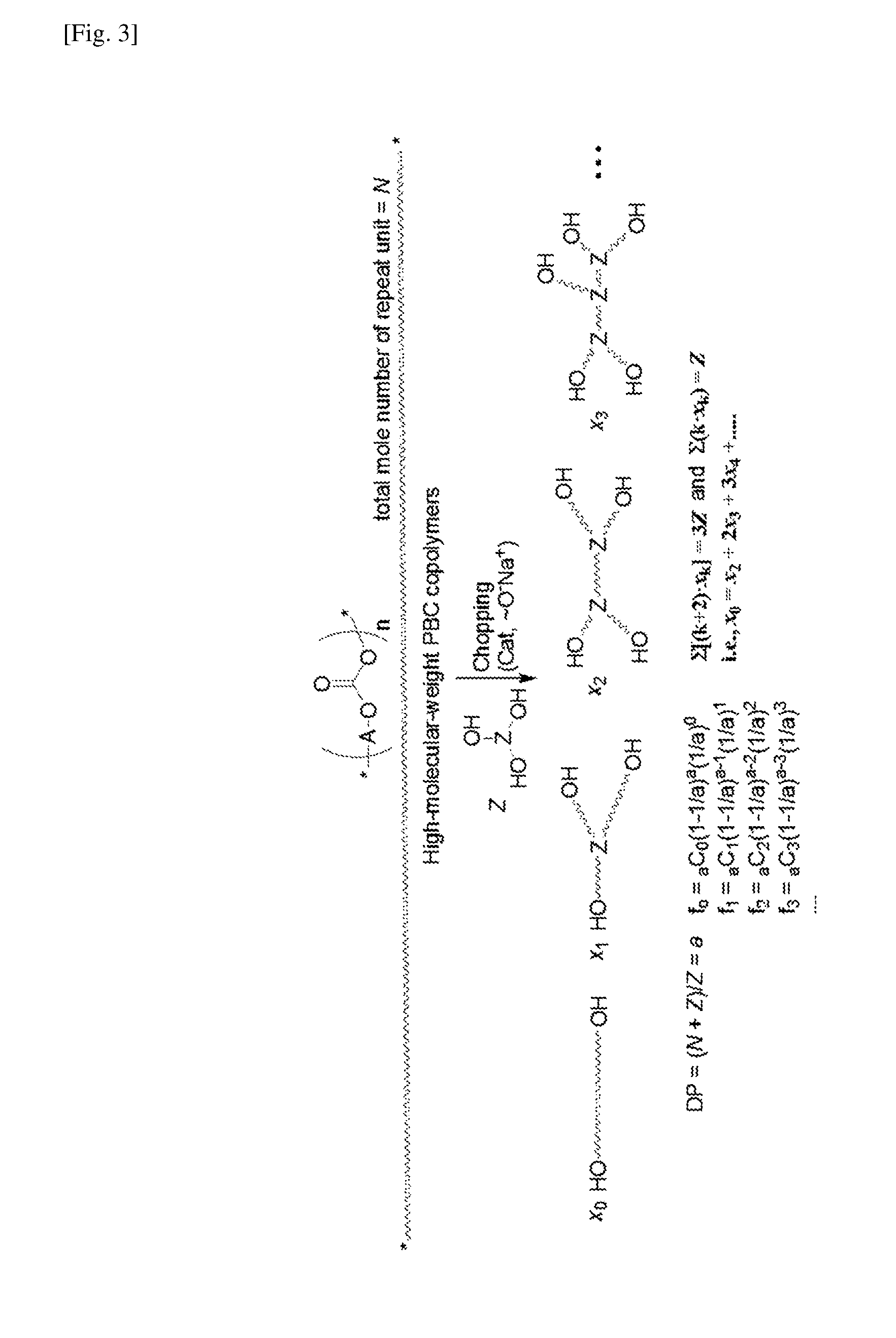
Aliphatic polycarbonate macropolyol and aliphatic polycarbonate-co-aromatic polyester macropolyol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-9
Condensation of Formula 1a (and Optionally Formula 1b or 1c) with DMC and Subsequent Chopping of the Condensation Product with One of Formulae 1b to 1h
[0050]First step: 1,4-Butanediol (Formula 1a) and optionally 1,5-pentanediol (Formula 1b) or 1,6-hexanediol (Formula 1c) were placed in a 3-neck flask such that the total number of moles was 111 mmol. The 1,5-pentanediol or 1,6-hexanediol was used in the amount of 0 or 10 mol %, as shown in Table 1. NaH (0.056 mmol, 0.05 mol %) was added to the flask to form HO(CH2)4O−Na+ and then 15.7 g (174 mmol) of dimethyl carbonate (DMC) was added thereto. A mechanical stirrer was connected to one neck of the flask, a manifold attached with a vacuum line and a nitrogen line was connected to another neck of the flask, and a distillation unit was connected to the remaining neck of the flask. After the reaction flask was immersed in a thermostatic bath at 120° C., the reaction was carried out for 1 h while distilling off formed methanol and a portio...
examples 10-21
Condensation of Formula 1a (and Optionally Formula 1c) with DMC and Subsequent Chopping of the Condensation Product with One of Formulae 2a to 2d
[0053]The first step of Examples 1-9 was repeated.
[0054]The second step of Examples 1-9 was repeated, except that a diol selected from Formulae 2a to 2d was used as a chopping agent. The experimental results are summarized in Table 2.
TABLE 2optionally Formula 1c) and dimethyl carbonate was chopped with one of Formulae 2a to 2d>Chopping agent,YieldaBefore chopping-After chopping-TgState after 1State after 7HOAOHZ(OH)a (15 mol %)(%)Mn (Mw / Mn)bMn (Mw / Mn)b(° C.)cdaydaysExample 101a2a8169000 (1.62)2200 (1.81)−49WaxWaxExample 111a2b8643200 (1.58)2100 (1.96)−45WaxWaxExample 121a2c8549900 (1.54)2000 (1.84)−57WaxWaxExample 131a2d8250000 (1.61)1300 (1.71)−41WaxWaxExample 141a + 1c2a8153000 (1.43)2100 (1.85)−50TransparentWax(95:5)oilExample 151a + 1c2b8787000 (1.63)2200 (1.87)−48TransparentSuspended(95:5)oiloilExample 161a + 1c2c8450000 (1.72)1000 (1....
examples 22-35
Condensation of Formula 1a and One of Formulae 1b to 1h with DMC and Subsequent Chopping of the Condensation Product with Formula 2a
[0056]First step: 1,4-Butanediol (Formula 1a) and an additional diol selected from Formulae 1b to 1h were placed in a 3-neck flask such that the total number of moles was 111 mmol. The additional diol was used in the amount of 5 mol % or 10 mol %, as shown in Table 1. NaH (0.056 mmol, 0.05 mol %) was added to the flask to form HO(CH2)4O−Na+ and then 15.7 g (174 mmol) of dimethyl carbonate (DMC) was added thereto. The subsequent procedure was carried out in the same manner as in Examples 1-9.
[0057]Second step: The triol of Formula 2a as a chopping agent was added to the condensation product obtained in the first step. The chopping agent was used in an amount of 15 mol % (4.43 g, 16.7 mmol), based on the diols initially added. The reaction was carried out for 3 h while slowly cooling to 150° C. from 190° C. Within 10 min from the beginning of the reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com